Biocatalytic Hydrogen-Borrowing Cascade in Organic Synthesis
- PMID: 38559715
- PMCID: PMC10976568
- DOI: 10.1021/jacsau.4c00026
Biocatalytic Hydrogen-Borrowing Cascade in Organic Synthesis
Abstract
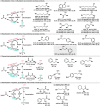
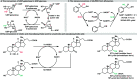
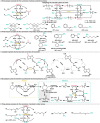
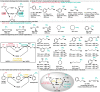
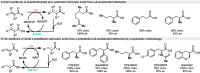
Biocatalytic hydrogen borrowing represents an environmentally friendly and highly efficient synthetic method. This innovative approach involves converting various substrates into high-value-added products, typically via a one-pot, two/three-step sequence encompassing dehydrogenation (intermediate transformation) and hydrogenation processes employing the hydride shuffling between NAD(P)+ and NAD(P)H. Represented key transformations in hydrogen borrowing include stereoisomer conversion within alcohols, conversion between alcohols and amines, conversion of allylic alcohols to saturated carbonyl counterparts, and α,β-unsaturated aldehydes to saturated carboxylic acids, etc. The direct transformation methodology and environmentally benign characteristics of hydrogen borrowing have contributed to its advancements in fine chemical synthesis or drug developments. Over the past decades, the hydrogen borrowing strategy in biocatalysis has led to the creation of diverse catalytic systems, demonstrating substantial potential for straightforward synthesis as well as asymmetric transformations. This perspective serves as a detailed exposition of the recent advancements in biocatalytic reactions employing the hydrogen borrowing strategy. It provides insights into the potential of this approach for future development, shedding light on its promising prospects in the field of biocatalysis.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

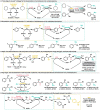

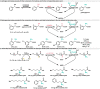
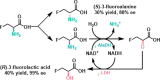
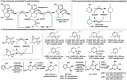
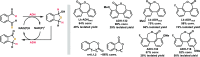
References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
