FeN4 Environments upon Reduction: A Computational Analysis of Spin States, Spectroscopic Properties, and Active Species
- PMID: 38559729
- PMCID: PMC10976608
- DOI: 10.1021/jacsau.3c00714
FeN4 Environments upon Reduction: A Computational Analysis of Spin States, Spectroscopic Properties, and Active Species
Abstract
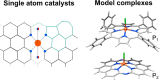
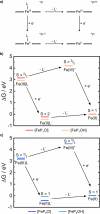
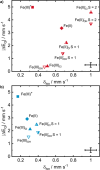
FeN4 motifs, found, for instance, in bioinorganic chemistry as heme-type cofactors, play a crucial role in man-made FeNC catalysts for the oxygen reduction reaction. Such single-atom catalysts are a potential alternative to platinum-based catalysts in fuel cells. Since FeNC catalysts are prepared via pyrolysis, the resulting materials are amorphous and contain side phases and impurities. Therefore, the geometric and electronic nature of the catalytically active FeN4 site remains to be clarified. To further understand the behavior of FeN4 centers in electrochemistry and their expected spectroscopic behavior upon reduction, we investigate two FeN4 environments (pyrrolic and pyridinic). These are represented by the model complexes [Fe(TPP)Cl] and [Fe(phen2N2)Cl], where TPP = tetraphenylporphyrin and phen = 1,10-phenanthroline. We predict their Mössbauer, UV-vis, and NRV spectral data using density functional theory as windows into their electronic structure differences. By varying the axial ligand, we further show how well small chemical changes in both complexes can be discerned. We find that the differences in ligand field strength in pyrrolic and pyridinic coordination result in different spin ground states, which in turn leads to distinct Mössbauer spectroscopic properties. As a result, pyrrolic nitrogen donors with a weaker ligand field are predicted to show more pronounced spectroscopic differences under in situ and operando conditions, while pyridinic nitrogen donors are expected to show less pronounced spectroscopic changes upon reduction and/or ligand loss. We therefore suggest that a weaker ligand field leads to better detectability of catalytic intermediates in in situ and operando experiments.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Haller S.; Gridin V.; Hofmann K.; Stark R. W.; Albert B.; Kramm U. I. Application of Non-Precious Bifunctional Catalysts for Metal-Air Batteries. Energy Tech 2021, 9 (7), 2001106. 10.1002/ente.202001106. - DOI
-
- Dodelet J.-P.Oxygen Reduction in PEM Fuel Cell Conditions: Heat-Treated Non-Precious Metal-N4Macrocycles and Beyond. In N4-Macrocyclic Metal Complexes; Zagal J. H., Bedioui F., Dodelet J.-P., Eds.; Springer New York: New York, NY, 2006, pp 83–147. 10.1007/978-0-387-28430-9_3. - DOI
-
- Zhang H.; Chung H. T.; Cullen D. A.; Wagner S.; Kramm U. I.; More K. L.; Zelenay P.; Wu G. High-Performance Fuel Cell Cathodes Exclusively Containing Atomically Dispersed Iron Active Sites. Energy Environ. Sci. 2019, 12 (8), 2548–2558. 10.1039/C9EE00877B. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
