Mitochondrial P-JNK target, SAB (SH3BP5), in regulation of cell death
- PMID: 38559813
- PMCID: PMC10978662
- DOI: 10.3389/fcell.2024.1359152
Mitochondrial P-JNK target, SAB (SH3BP5), in regulation of cell death
Abstract
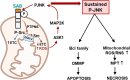
Cell death occurs in various circumstances, such as homeostasis, stress response, and defense, via specific pathways and mechanisms that are regulated by specific activator-induced signal transductions. Among them, Jun N-terminal kinases (JNKs) participate in various aspects, and the recent discovery of JNKs and mitochondrial protein SAB interaction in signal regulation of cell death completes our understanding of the mechanism of sustained activation of JNK (P-JNK), which leads to triggering of the machinery of cell death. This understanding will lead the investigators to discover the modulators facilitating or preventing cell death for therapeutic application in acute or chronic diseases and cancer. We discuss here the mechanism and modulators of the JNK-SAB-ROS activation loop, which is the core component of mitochondria-dependent cell death, specifically apoptosis and mitochondrial permeability transition (MPT)-driven necrosis, and which may also contribute to cell death mechanisms of ferroptosis and pyroptosis. The discussion here is based on the results and evidence discovered from liver disease models, but the JNK-SAB-ROS activation loop to sustain JNK activation is universally applicable to various disease models where mitochondria and reactive oxygen species contribute to the mechanism of disease.
Keywords: JNK-SAB-ROS activation loop; Jun N-terminal kinase; SAB (SH3BP5); SAB-KIM1 peptide; apoptosis; ferroptosis; necrosis; pyroptosis.
Copyright © 2024 Win, Than and Kaplowitz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

