Structural and Electronic Insights into 1-Ethyl-3-Methylimidazolium Bis(fluorosulfonyl)imide Ion Pair Conformers: Ab Initio DFT Study
- PMID: 38559957
- PMCID: PMC10975623
- DOI: 10.1021/acsomega.4c00104
Structural and Electronic Insights into 1-Ethyl-3-Methylimidazolium Bis(fluorosulfonyl)imide Ion Pair Conformers: Ab Initio DFT Study
Abstract
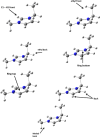
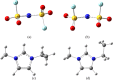
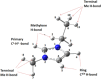
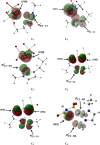
An understanding of the nature of molecular interactions among the ion pairs of 1-ethyl-3-methylimidazolium bis(fluorosulfonyl)imide [EMI[FSI]] can offer a starting point and significant insight into the more dynamic and multiple interactions within the bulk liquid state. In this context, close inspection of ion pair conformers can offer insight into the effects in bulk [EMI][FSI] liquid. The current work, therefore, gives a detailed analysis of the [EMI][FSI] ion pair conformers through analysis of the interaction energies, stabilization energies, and natural orbital of the ion pair conformers. The structures of the cations, anions, and cation-anion ion pairs of the conformers are optimized systematically by the ωB97X-D method with the DGDZVP basis sets, considering both the empirical dispersion corrections and the presence of a polar solvent, and the most stable geometries are obtained. The [FSI]- anions, unlike [TFSI]- anions, exist at the top position with respect to imidazolium rings. The presence of out-of-plane interactions between the [EMI]+ and [FSI]- ions is in good agreement with the stronger interactions of the [FSI]- anions with alkyl group hydrogens. The presence of out-of-plane conformers could also be related to the interaction of the anion with the π clouds of the [EMI]+ ring. In the [EMI]+ cation, the aromatic ring is π-acidic due to the presence of a positive charge in the N1-C1-N2 ring, which leads to the presence of [FSI]- anion donor [EMI]+ π-acceptor type interactions. The [EMI]+ cation and [FSI]- anions tend to form multiple σ* and π* interactions but reduce the strength of the individual contributions from a potential (linear) maximum. For the ion pair [EMI][FSI], the absolute value of the interaction energies is lower than the normal hydrogen bond energy (50 kJ/mol), which indicates that there is a very weak electrostatic interaction between the [EMI]+ cations and [FSI]- anions. The weaker attraction between the [EMI]+ and [FSI]- ions is suggested to contribute to the larger diffusion coefficients of the ions.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Anion conformation of low-viscosity room-temperature ionic liquid 1-ethyl-3-methylimidazolium bis(fluorosulfonyl) imide.J Phys Chem B. 2007 Nov 8;111(44):12829-33. doi: 10.1021/jp074325e. Epub 2007 Oct 17. J Phys Chem B. 2007. PMID: 17941662
-
Molecular dynamics simulation and pulsed-field gradient NMR studies of bis(fluorosulfonyl)imide (FSI) and bis[(trifluoromethyl)sulfonyl]imide (TFSI)-based ionic liquids.J Phys Chem B. 2010 May 27;114(20):6786-98. doi: 10.1021/jp911950q. J Phys Chem B. 2010. PMID: 20433203
-
Ab Initio Force Fields for Organic Anions: Properties of [BMIM][TFSI], [BMIM][FSI], and [BMIM][OTf] Ionic Liquids.J Phys Chem B. 2018 Apr 12;122(14):4101-4114. doi: 10.1021/acs.jpcb.8b01221. Epub 2018 Mar 23. J Phys Chem B. 2018. PMID: 29536738
-
Liquid structure of room-temperature ionic liquid, 1-Ethyl-3-methylimidazolium bis-(trifluoromethanesulfonyl) imide.J Phys Chem B. 2008 Apr 10;112(14):4329-36. doi: 10.1021/jp7105499. Epub 2008 Mar 19. J Phys Chem B. 2008. PMID: 18348563
-
Inter- and Intramolecular Interactions in Ether-Functionalized Ionic Liquids.J Phys Chem B. 2021 Mar 11;125(9):2380-2388. doi: 10.1021/acs.jpcb.0c11429. Epub 2021 Feb 24. J Phys Chem B. 2021. PMID: 33625218
References
-
- Wang Q.; Ping P.; Zhao X.; Chu G.; Sun J.; Chen C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. 10.1016/j.jpowsour.2012.02.038. - DOI
-
- Rogers J. R. D.; Seddon K. R.. Ionic Liquids: Industrial Application to Green Chemistry, ACS Symposium Series; ACS Publications: Washington, 2002; Vol. 818.
-
- Ohno H.Electrochemical Aspects of Ionic Liquids; John Wiley & Sons Inc.: Hoboken, NJ, 2005.
-
- Sayah S.; Ghamouss F.; Santos-Peña J.; Tran-Van F.; Lemordant D. The Intriguing Properties of 1-Ethyl-3-methylimidazolium bis(fuorosulfonyl)imide Ionic Liquid. J. Solution Chem. 2019, 48, 992–1008. 10.1007/s10953-018-0814-0. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
