First-Principles Investigations of Novel Guanidine-Based Dyes
- PMID: 38559970
- PMCID: PMC10976409
- DOI: 10.1021/acsomega.3c09182
First-Principles Investigations of Novel Guanidine-Based Dyes
Abstract
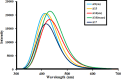
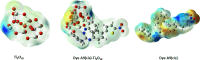
In the pursuit of finding efficient D-π-A organic dyes as photosensitizers for dye-sensitized solar cells (DSSCs), first-principles calculations of guanidine-based dyes [A1-A18] were executed using density functional theory (DFT). The various electronic and optical properties of guanidine-based organic dyes with different D-π-A structural modifications were investigated. The structural modification of guanidine-based dyes largely affects the properties of molecules, such as excitation energies, the oscillator strength dipole moment, the transition dipole moment, and light-harvesting efficiencies. The energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) is responsible for the reduction and injection of electrons. Modification of the guanidine subunit by different structural modifications gave a range of HOMO-LUMO energy gaps. Chemical and optical characteristics of the dyes indicated prominent charge transfer and light-harvesting efficiencies. The wide electronic absorption spectra of these guanidine-based dyes computed by TD-DFT-B3LYP with 6-31G, 6-311G, and cc-PVDZ basis sets have been observed in the visible region of spectra due to the presence of chromophore groups of dye molecules. Better anchorage of dyes to the surface of TiO2 semiconductors helps in charge-transfer phenomena, and the results suggested that -COOH, -CN, and -NO2 proved to be proficient anchoring groups, making dyes very encouraging candidates for DSSCs. Molecular electrostatic potential explained the electrostatic potential of organic dyes, and IR spectrum and conformational analyses ensured the suitability of organic dyes for the fabrication of DSSCs.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Synergistic charge-transfer dynamics of novel D-D-A-π-A framework containing indoline-benzo[d][1,2,3]thiadiazole based push-pull sensitizers: from structural engineering to performance metrics in photovoltaic solar cells.J Mol Model. 2024 Sep 17;30(10):338. doi: 10.1007/s00894-024-06140-7. J Mol Model. 2024. PMID: 39287837
-
Tuning the charge transfer and optoelectronic properties of tetrathiafulvalene based organic dye-sensitized solar cells: a theoretical approach.RSC Adv. 2021 Dec 8;11(62):39246-39261. doi: 10.1039/d1ra05887h. eCollection 2021 Dec 6. RSC Adv. 2021. PMID: 35492466 Free PMC article.
-
Designed complexes combining brazilein and brazilin with betanidin for dye-sensitized solar cell application: DFT and TD-DFT study.J Mol Graph Model. 2024 Mar;127:108691. doi: 10.1016/j.jmgm.2023.108691. Epub 2023 Dec 6. J Mol Graph Model. 2024. PMID: 38086144
-
Hydrazone-based Materials; DFT, TD-DFT, NBO Analysis, Fukui Function, MESP Analysis, and Solar Cell Applications.J Fluoresc. 2022 Sep;32(5):1857-1871. doi: 10.1007/s10895-022-03000-6. Epub 2022 Jun 23. J Fluoresc. 2022. PMID: 35737283 Free PMC article. Review.
-
Coupled Polymethine Dyes: Six Decades of Discoveries.Chem Rec. 2024 Dec;24(12):e202400183. doi: 10.1002/tcr.202400183. Epub 2024 Nov 11. Chem Rec. 2024. PMID: 39529436 Review.
Cited by
-
Microwave-Assisted Synthesis of Near-Infrared Chalcone Dyes: a Systematic Approach.ACS Omega. 2025 Feb 13;10(7):7317-7326. doi: 10.1021/acsomega.4c11066. eCollection 2025 Feb 25. ACS Omega. 2025. PMID: 40028138 Free PMC article.
References
-
- O’regan B.; Grätzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. nature 1991, 353 (6346), 737.10.1038/353737a0. - DOI
-
- Nazeeruddin M. K.; Pechy P.; Renouard T.; Zakeeruddin S. M.; Humphry-Baker R.; Comte P.; Liska P.; Cevey L.; Costa E.; Shklover V. Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. J. Am. Chem. Soc. 2001, 123 (8), 1613–1624. 10.1021/ja003299u. - DOI - PubMed
-
- Hara K.; Sato T.; Katoh R.; Furube A.; Yoshihara T.; Murai M.; Kurashige M.; Ito S.; Shinpo A.; Suga S. Novel conjugated organic dyes for efficient dye-sensitized solar cells. Adv. Funct. Mater. 2005, 15 (2), 246–252. 10.1002/adfm.200400272. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
