Efficacy and safety of inclisiran a newly approved FDA drug: a systematic review and pooled analysis of available clinical studies
- PMID: 38560059
- PMCID: PMC10978220
- DOI: 10.1016/j.ahjo.2022.100127
Efficacy and safety of inclisiran a newly approved FDA drug: a systematic review and pooled analysis of available clinical studies
Abstract
Study objective: This systematic review and meta-analysis aimed to assess the efficacy and safety profile of treatment with inclisiran, a drug that has been recently approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
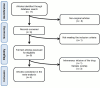
Design: A systematic literature search was conducted in order to identify randomized controlled trials (RCTs) assessing the effect on lipoproteins and the safety profile of inclisiran.
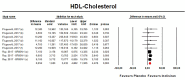
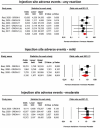
Results: Data were pooled from 5 RCTs, which included 4226 subjects. Meta-analyses of data suggested that the multiple-dose regimens of inclisiran yielded a significant reduction in serum levels of proprotein convertase subtilisin/kexin type 9 (MD = -78.23%, 95%CI: -86.74, -69.71) and low-density lipoprotein cholesterol (MD = -45.48%, 95%CI: -50.36%, -40.61%) throughout the studies. Furthermore, treatment with inclisiran significantly affected total cholesterol (MD = -13.67%, 95%CI: -20.78%, -6.57%), high-density lipoprotein cholesterol (MD = 8.29%, 95%CI: 4.66%,11.93%), non-HDL cholesterol (MD = -39.45%, 95%CI: -43.6%, -35.31%), apolipoprotein B (MD = -34.58%, 95%CI: -38.78%, -30.78%) and lipoprotein(a) (MD = -20.9%, 95%CI: -25.8%, -15.99%). Multiple-dose regimens of inclisiran were associated with increased risk of injection-site reactions (any reaction: OR = 5.86, 95%CI: 3.44, 9.98; mild reactions: OR = 5.19, 95%CI: 1.68, 16.07; moderate reactions: OR = 13.37, 95%CI: 3.17, 56.46), and bronchitis (OR = 1.58, 95%CI: 1.10, 2.26), while the incidence of the pre-specified exploratory CV endpoint significantly decreased at 18 months (OR = 0.74, 95%CI: 0.58, 0.94).
Conclusion and relevance: Inclisiran has favourable effects on serum lipid levels and an acceptable safety profile. Further well-designed RCTs are needed to explore its longer-term safety.
Keywords: Hypercholesterolemia;lipoprotein(a); Inclisiran; Low-density lipoprotein cholesterol; Proprotein convertase subtilisin/kexin type 9; Silencing ribonucleic acid.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Ference B.A., Ginsberg H.N., Graham I., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017;38:2459–2472. - PMC - PubMed
-
- ESC Committee for Practice Guidelines (CPG) ESC National Cardiac Societies 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. - PubMed
-
- Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the Management of blood cho-lesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2019;73:e285–e350. - PubMed
-
- Reiner Ž. Statins in the primary prevention of cardiovascular disease. Nat. Rev. Cardiol. 2013;10:453–464. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

