Clinical Burden of Chronic Obstructive Pulmonary Disease in Patients with Suboptimal Peak Inspiratory Flow
- PMID: 38560416
- PMCID: PMC10980549
- DOI: 10.1155/2024/8034923
Clinical Burden of Chronic Obstructive Pulmonary Disease in Patients with Suboptimal Peak Inspiratory Flow
Abstract
Introduction: Many patients with chronic obstructive pulmonary disease (COPD) may derive inadequate benefit from dry powder inhalers (DPIs) because of suboptimal peak inspiratory flow (sPIF).
Objectives: To assess the clinical burden of COPD by characterizing the clinical characteristics of participants with sPIF against medium-low resistance DPIs versus those with optimal PIF (oPIF) from two phase 3 clinical trials.
Methods: Baseline data were collected from two randomized, controlled, phase 3 trials (NCT03095456; NCT02518139) in participants with moderate-to-severe COPD. oPIF (60 L/min) against the medium-low resistance DPIs was used as the threshold for defining the PIF subgroups (<60 L/min (sPIF) vs ≥60 L/min (oPIF)).
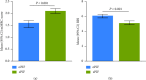
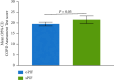
Results: Most participants included in this analysis were White (92%) and male (63%); the mean (range) age was 65 (43-87) years. Participants with sPIF had significantly greater dyspnea than those with oPIF as measured using the modified Medical Research Council scoring (mean (95% CI): 2.1 (2.0-2.2) vs 1.6 (1.4-1.7); P < 0.001) and baseline dyspnea index (mean (95% CI): 5.1 (4.9-5.4) vs 6.1 (5.8-6.3); P < 0.001). Based on COPD Assessment Test scores, participants with sPIF had a higher COPD symptom burden than those with oPIF (mean (95% CI): 21.5 (19.7-23.3) vs 19.5 (18.6-20.4); P = 0.05).
Conclusion: In these trials, participants with COPD who had sPIF against the medium-low resistance DPIs had more dyspnea and worse health status than those with oPIF. These results demonstrate that sPIF is associated with a higher clinical burden as measured by patient-reported outcomes.
Copyright © 2024 Jill A. Ohar et al.
Conflict of interest statement
JAO has served on advisory boards for AstraZeneca, Boehringer Ingelheim, GSK, Mylan Inc., Reckitt Benckiser, Sunovion Pharmaceuticals, and Theravance Biopharma US, Inc. DAM has served on advisory boards for AstraZeneca, Boehringer Ingelheim, GSK, Mylan, Sunovion Pharmaceuticals, Theravance Biopharma US, Inc., and Teva and is on the speaker's bureau for AstraZeneca, Boehringer Ingelheim, and Teva Pharmaceuticals. GND is an employee of Theravance Biopharma US, Inc. DAL is a contract employee of Theravance Biopharma US, Inc. EJM is an employee of Theravance Biopharma US, Inc. GDC was an employee of Theravance Biopharma US, Inc., at the time these trials were conducted.
Figures

References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2024 report. https://goldcopd.org/2024-gold-report/
-
- Duarte A. G., Tung L., Zhang W., Hsu E. S., Kuo Y. F., Sharma G. Spirometry measurement of peak inspiratory flow identifies suboptimal use of dry powder inhalers in ambulatory patients with COPD. Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation . 2019;6(3):246–255. doi: 10.15326/jcopdf.6.3.2018.0163. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

