DNA-delivered monoclonal antibodies targeting the p53 R175H mutant epitope inhibit tumor development in mice
- PMID: 38560504
- PMCID: PMC10980946
- DOI: 10.1016/j.gendis.2023.04.027
DNA-delivered monoclonal antibodies targeting the p53 R175H mutant epitope inhibit tumor development in mice
Abstract
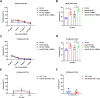
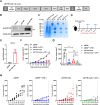
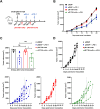
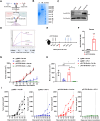
The tumor suppressor p53 is the most common mutated gene in cancer, with the R175H as the most frequent p53 missense mutant. However, there are currently no approved targeted therapies or immunotherapies against mutant p53. Here, we characterized and investigated a monoclonal antibody (mAb) that recognizes the mutant p53-R175H for its affinity, specificity, and activity against tumor cells in vitro. We then delivered DNA plasmids expressing the anti-R175H mAb or a bispecific antibody (BsAb) into mice to evaluate their therapeutic effects. Our results showed that the anti-R175H mAb specifically bound to the p53-R175H antigen with a high affinity and recognized the human mutant p53-R175H antigen expressed on HEK293T or MC38 cells, with no cross-reactivity with wild-type p53. In cultured cells, the anti-R175H mAb showed higher cytotoxicity than the control but did not induce antibody-dependent cellular cytotoxicity. We made a recombinant MC38 mouse cell line (MC38-p53-R175H) that overexpressed the human p53-R175H after knocking out the endogenous mutant p53 alleles. In vivo, administration of the anti-R175H mAb plasmid elicited a robust anti-tumor effect against MC38-p53-R175H in mice. The administration of the anti-R175H BsAb plasmid showed no therapeutic effects, yet potent anti-tumor activity was observed in combination with the anti-PD-1 antibody. These results indicate that targeting specific mutant epitopes using DNA-delivered mAbs or BsAbs presents a form of improved natural immunity derived from tumor-infiltrating B cells and plasma cells against intracellular tumor antigens.
Keywords: BsAb; Cytotoxicity; Mutant p53; PD-1; R175H; mAb.
© 2023 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Figures

Similar articles
-
Targeting mutant p53: Evaluation of novel anti-p53R175H monoclonal antibodies as diagnostic tools.Sci Rep. 2025 Jan 6;15(1):1000. doi: 10.1038/s41598-024-83871-w. Sci Rep. 2025. PMID: 39762369 Free PMC article.
-
The Function of the Mutant p53-R175H in Cancer.Cancers (Basel). 2021 Aug 13;13(16):4088. doi: 10.3390/cancers13164088. Cancers (Basel). 2021. PMID: 34439241 Free PMC article. Review.
-
Differential effect of plakoglobin in restoring the tumor suppressor activities of p53-R273H vs. p53-R175H mutants.PLoS One. 2024 Oct 3;19(10):e0306705. doi: 10.1371/journal.pone.0306705. eCollection 2024. PLoS One. 2024. PMID: 39361615 Free PMC article.
-
The gain of function of p53 cancer mutant in promoting mammary tumorigenesis.Oncogene. 2013 Jun 6;32(23):2900-6. doi: 10.1038/onc.2012.299. Epub 2012 Jul 23. Oncogene. 2013. PMID: 22824795 Free PMC article.
-
Full-length recombinant antibodies from Escherichia coli: production, characterization, effector function (Fc) engineering, and clinical evaluation.MAbs. 2022 Jan-Dec;14(1):2111748. doi: 10.1080/19420862.2022.2111748. MAbs. 2022. PMID: 36018829 Free PMC article. Review.
Cited by
-
Mutant p53 Gain of Function: Why Many See It, Why Some Do Not.Cancer Discov. 2025 Jun 3;15(6):1099-1104. doi: 10.1158/2159-8290.CD-24-1638. Cancer Discov. 2025. PMID: 40287981
-
Remodeling of anti-tumor immunity with antibodies targeting a p53 mutant.J Hematol Oncol. 2024 Jun 18;17(1):45. doi: 10.1186/s13045-024-01566-1. J Hematol Oncol. 2024. PMID: 38886748 Free PMC article.
-
Expanding nucleic acid-encoded medicine.Mol Ther. 2025 Jan 8;33(1):16-17. doi: 10.1016/j.ymthe.2024.12.023. Epub 2024 Dec 20. Mol Ther. 2025. PMID: 39708802 No abstract available.
-
Efficacy analysis of targeted P53 therapy in solid tumors.Med Oncol. 2025 Jul 22;42(8):360. doi: 10.1007/s12032-025-02930-y. Med Oncol. 2025. PMID: 40694182 Review.
References
-
- Finlay C.A., Hinds P.W., Levine A.J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57(7):1083–1093. - PubMed
-
- Levine A.J., Finlay C.A., Hinds P.W. P53 is a tumor suppressor gene. Cell. 2004;116(2 Suppl):S67–S69. - PubMed
-
- Bouaoun L., Sonkin D., Ardin M., et al. TP53 variations in human cancers: new lessons from the IARC TP53 database and genomics data. Hum Mutat. 2016;37(9):865–876. - PubMed
-
- Goh A.M., Coffill C.R., Lane D.P. The role of mutant p53 in human cancer. J Pathol. 2011;223(2):116–126. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

