Membrane-assisted tariquidar access and binding mechanisms of human ATP-binding cassette transporter P-glycoprotein
- PMID: 38560519
- PMCID: PMC10979361
- DOI: 10.3389/fmolb.2024.1364494
Membrane-assisted tariquidar access and binding mechanisms of human ATP-binding cassette transporter P-glycoprotein
Abstract
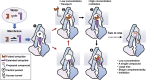
The human multidrug transporter P-glycoprotein (P-gp) is physiologically essential and of key relevance to biomedicine. Recent structural studies have shed light on the mode of inhibition of the third-generation inhibitors for human P-gp, but the molecular mechanism by which these inhibitors enter the transmembrane sites remains poorly understood. In this study, we utilized all-atom molecular dynamics (MD) simulations to characterize human P-gp dynamics under a potent inhibitor, tariquidar, bound condition, as well as the atomic-level binding pathways in an explicit membrane/water environment. Extensive unbiased simulations show that human P-gp remains relatively stable in tariquidar-free and bound states, while exhibiting a high dynamic binding mode at either the drug-binding pocket or the regulatory site. Free energy estimations by partial nudged elastic band (PNEB) simulations and Molecular Mechanics Generalized Born Surface Area (MM/GBSA) method identify two energetically favorable binding pathways originating from the cytoplasmic gate with an extended tariquidar conformation. Interestingly, free tariquidar in the lipid membrane predominantly adopts extended conformations similar to those observed at the regulatory site. These results suggest that membrane lipids may preconfigure tariquidar into an active ligand conformation for efficient binding to the regulatory site. However, due to its conformational plasticity, tariquidar ultimately moves toward the drug-binding pocket in both pathways, explaining how it acts as a substrate at low concentrations. Our molecular findings propose a membrane-assisted mechanism for the access and binding of the third-generation inhibitors to the binding sites of human P-gp, and offer deeper insights into the molecule design of more potent inhibitors against P-gp-mediated drug resistance.
Keywords: human P-glycoprotein; mechanism of action; membrane lipids; molecular dynamics simulations; tariquidar; third-generation inhibitors.
Copyright © 2024 Gao, Wei, Luo, Tang, Yu, Li, Xing and Pan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Bankstahl J. P., Bankstahl M., Romermann K., Wanek T., Stanek J., Windhorst A. D., et al. (2013). Tariquidar and elacridar are dose-dependently transported by P-glycoprotein and Bcrp at the blood-brain barrier: a small-animal positron emission tomography and in vitro study. Drug Metab. Dispos. 41 (4), 754–762. 10.1124/dmd.112.049148 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

