Propolis-loaded liposomes: characterization and evaluation of the in vitro bioaccessibility of phenolic compounds
- PMID: 38560718
- PMCID: PMC10974815
- DOI: 10.5599/admet.2204
Propolis-loaded liposomes: characterization and evaluation of the in vitro bioaccessibility of phenolic compounds
Abstract
Background and purpose: Propolis has low water solubility, poor stability, and limited bioaccessibility of phenolic constituents when subjected to in vitro digestion. To overcome these drawbacks, the liposomal encapsulation method can be employed.
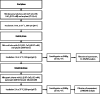
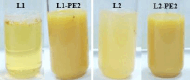
Experimental approach: Soybean phosphatidylcholine lecithin mixed with Tween 80 (T80) and ammonium phosphatides (AMP) was used to produce propolis extract (PE)-loaded liposomes. The mean particle size, zeta potential, encapsulation efficiency values, and transmission electron microscopy analysis were used to characterize liposomes. Individual phenolics were determined for digested and nondigested propolis-loaded liposomes and propolis extract.
Key results: Tween 80 incorporation reduced the size of unloaded liposomes, whereas AMP inclusion yielded larger liposomes. In both formulations, PE loading significantly increased the size and reduced the zeta potential values and homogeneity of the size distribution. In free PE, the most bioaccessible polyphenols were phenolic acids (3.20 to 5.63 %), and flavonoids such as caffeic acid phenethyl ester, galangin, pinobanksin, and pinocembrin (0.03 to 2.12 %) were the least bioaccessible. Both liposomal propolis provided significantly higher bioaccessibility of phenolic compounds. The liposomes with T80 and AMP in their compositions recovered 52.43 and 185.90 % of the total amount of phenolic compounds in the nondigested samples, respectively. The liposomes containing AMP not only exhibited high solubility for PE but also provided protection to the phenolic compounds during in vitro digestion.
Conclusion: Liposomal encapsulation could be a promising approach to improving the solubility and stability of PE in digestive fluids, making it suitable for the delivery of propolis in oral formulations.
Keywords: Ammonium phosphatides; Tween 80; encapsulation; flavonoids; in vitro digestion.
Copyright © 2024 by the authors.
Conflict of interest statement
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Liposomal propolis loaded xanthan gum-salep hydrogels: Preparation, characterization, and in vitro bioaccessibility of phenolics.Int J Biol Macromol. 2025 Apr;300:140323. doi: 10.1016/j.ijbiomac.2025.140323. Epub 2025 Jan 24. Int J Biol Macromol. 2025. PMID: 39864705
-
Investigation of antioxidant capacity, bioaccessibility and LC-MS/MS phenolic profile of Turkish propolis.Food Res Int. 2019 Aug;122:528-536. doi: 10.1016/j.foodres.2019.05.028. Epub 2019 May 21. Food Res Int. 2019. PMID: 31229108
-
The evaluation of L-arginine solution as a solvent for propolis extraction: The phenolic profile, antioxidant, antibacterial activity, and in vitro bioaccessibility.Food Sci Nutr. 2024 Jan 11;12(4):2724-2735. doi: 10.1002/fsn3.3953. eCollection 2024 Apr. Food Sci Nutr. 2024. PMID: 38628177 Free PMC article.
-
Encapsulation of phenolic compounds with liposomal improvement in the cosmetic industry.Int J Pharm. 2021 Jan 25;593:120125. doi: 10.1016/j.ijpharm.2020.120125. Epub 2020 Nov 28. Int J Pharm. 2021. PMID: 33253799 Review.
-
Propolis: Its Role and Efficacy in Human Health and Diseases.Molecules. 2022 Sep 19;27(18):6120. doi: 10.3390/molecules27186120. Molecules. 2022. PMID: 36144852 Free PMC article. Review.
References
-
- Javed S., Mangla B., Ahsan W.. From propolis to nanopropolis: An exemplary journey and a paradigm shift of a resinous substance produced by bees. Phytotherapy Research 36 (2022) 2016-2041. https://doi.org/10.1002/ptr.7435 10.1002/ptr.7435 - DOI - PubMed
-
- Guzelmeric E., Sipahi H., Özhan Y., Hamitoğlu M., Helvacıoğlu S., Düz G., Akyıldız İ.E., Yaman B.K., Hazar M., Dilsiz S.A., Aydın A., Yesilada E.. Comprehensive estrogenic/anti-estrogenic, anticancer, mutagenic/anti-mutagenic, and genotoxic/anti-genotoxic activity studies on chemically characterized black poplar and Eurasian aspen propolis types. Journal of Pharmaceutical and Biomedical Analysis 226 (2023) 115241. https://doi.org/10.1016/j.jpba.2023.115241 10.1016/j.jpba.2023.115241 - DOI - PubMed
-
- Zhang H., Fu Y., Xu Y., Niu F., Li Z., Ba C., Jin B., Chen G., Li X.. One-step assembly of zein/caseinate/alginate nanoparticles for encapsulation and improved bioaccessibility of propolis. Food and Function 10 (2019) 635-645. https://doi.org/10.1039/c8fo01614c 10.1039/c8fo01614c - DOI - PubMed
-
- Ozdal T., Ceylan F.D., Eroglu N., Kaplan M., Olgun E.O., Capanoglu E.. Investigation of antioxidant capacity, bioaccessibility and LC-MS/MS phenolic profile of Turkish propolis. Food Research International 122 (2019) 528-536. https://doi.org/10.1016/j.foodres.2019.05.028 10.1016/j.foodres.2019.05.028 - DOI - PubMed
-
- Atayoglu A.T., Sözeri Atik D., Bölük E., Gürbüz B., Ceylan F.D., Çapanoğlu E., Atayolu R., Paradkar A., Fearnley J., Palabiyik I.. Evaluating bioactivity and bioaccessibility properties of the propolis extract prepared with L-lactic acid: An alternative solvent to ethanol for propolis extraction. Food Bioscience 53 (2023) 102756. https://doi.org/10.1016/j.fbio.2023.102756 10.1016/j.fbio.2023.102756 - DOI
LinkOut - more resources
Full Text Sources
