Pyruvate Kinase M2: A Potential Regulator of Cardiac Injury Through Glycolytic and Non-glycolytic Pathways
- PMID: 38560918
- PMCID: PMC11230662
- DOI: 10.1097/FJC.0000000000001568
Pyruvate Kinase M2: A Potential Regulator of Cardiac Injury Through Glycolytic and Non-glycolytic Pathways
Abstract
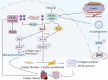
Adult animals are unable to regenerate heart cells due to postnatal cardiomyocyte cycle arrest, leading to higher mortality rates in cardiomyopathy. However, reprogramming of energy metabolism in cardiomyocytes provides a new perspective on the contribution of glycolysis to repair, regeneration, and fibrosis after cardiac injury. Pyruvate kinase (PK) is a key enzyme in the glycolysis process. This review focuses on the glycolysis function of PKM2, although PKM1 and PKM2 both play significant roles in the process after cardiac injury. PKM2 exists in both low-activity dimer and high-activity tetramer forms. PKM2 dimers promote aerobic glycolysis but have low catalytic activity, leading to the accumulation of glycolytic intermediates. These intermediates enter the pentose phosphate pathway to promote cardiomyocyte proliferation and heart regeneration. Additionally, they activate adenosine triphosphate (ATP)-sensitive K + (K ATP ) channels, protecting the heart against ischemic damage. PKM2 tetramers function similar to PKM1 in glycolysis, promoting pyruvate oxidation and subsequently ATP generation to protect the heart from ischemic damage. They also activate KDM5 through the accumulation of αKG, thereby promoting cardiomyocyte proliferation and cardiac regeneration. Apart from glycolysis, PKM2 interacts with transcription factors like Jmjd4, RAC1, β-catenin, and hypoxia-inducible factor (HIF)-1α, playing various roles in homeostasis maintenance, remodeling, survival regulation, and neovascularization promotion. However, PKM2 has also been implicated in promoting cardiac fibrosis through mechanisms like sirtuin (SIRT) 3 deletion, TG2 expression enhancement, and activation of transforming growth factor-β1 (TGF-β1)/Smad2/3 and Jak2/Stat3 signals. Overall, PKM2 shows promising potential as a therapeutic target for promoting cardiomyocyte proliferation and cardiac regeneration and addressing cardiac fibrosis after injury.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


Similar articles
-
AARS2 ameliorates myocardial ischemia via fine-tuning PKM2-mediated metabolism.Elife. 2025 May 15;13:RP99670. doi: 10.7554/eLife.99670. Elife. 2025. PMID: 40371904 Free PMC article.
-
Checkpoint Kinase 1 Stimulates Endogenous Cardiomyocyte Renewal and Cardiac Repair by Binding to Pyruvate Kinase Isoform M2 C-Domain and Activating Cardiac Metabolic Reprogramming in a Porcine Model of Myocardial Ischemia/Reperfusion Injury.J Am Heart Assoc. 2024 Jul 2;13(13):e034805. doi: 10.1161/JAHA.124.034805. Epub 2024 Jun 27. J Am Heart Assoc. 2024. PMID: 38934866 Free PMC article.
-
Sanqi oral solution ameliorates renal fibrosis by suppressing fibroblast activation via HIF-1α/PKM2/glycolysis pathway in chronic kidney disease.J Ethnopharmacol. 2024 Dec 5;335:118679. doi: 10.1016/j.jep.2024.118679. Epub 2024 Aug 8. J Ethnopharmacol. 2024. PMID: 39121930
-
Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells.Int J Biochem Cell Biol. 2011 Jul;43(7):969-80. doi: 10.1016/j.biocel.2010.02.005. Epub 2010 Feb 13. Int J Biochem Cell Biol. 2011. PMID: 20156581 Review.
-
The Role of PKM2 in Metabolic Reprogramming: Insights into the Regulatory Roles of Non-Coding RNAs.Int J Mol Sci. 2021 Jan 25;22(3):1171. doi: 10.3390/ijms22031171. Int J Mol Sci. 2021. PMID: 33503959 Free PMC article. Review.
Cited by
-
Regulation of heart regeneration by LSD1 through suppressing CEND1.Theranostics. 2025 May 25;15(13):6313-6328. doi: 10.7150/thno.110297. eCollection 2025. Theranostics. 2025. PMID: 40521201 Free PMC article.
-
Nomograms based on ratio indexes to predict severity and prognosis in immune checkpoint inhibitors-related myocarditis: a retrospective analysis.J Cancer Res Clin Oncol. 2024 May 27;150(5):277. doi: 10.1007/s00432-024-05801-7. J Cancer Res Clin Oncol. 2024. PMID: 38801421 Free PMC article.
-
PKM2-mediated metabolic reprogramming of microglia in neuroinflammation.Cell Death Discov. 2025 Apr 6;11(1):149. doi: 10.1038/s41420-025-02453-5. Cell Death Discov. 2025. PMID: 40189596 Free PMC article. Review.
References
-
- Writing Group Members, Mozaffarian D, Benjamin EJ, et al. . Heart disease and stroke statistics–2016 update: a report from the American heart association. Circulation. 2016;133:e38–e360. - PubMed
-
- Ribeiro dos Santos R, Rassi S, Feitosa G, et al. . Cell therapy in Chagas cardiomyopathy (Chagas arm of the multicenter randomized trial of cell therapy in cardiopathies study): a multicenter randomized trial. Circulation. 2012;125:2454–2461. - PubMed
-
- Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5:1841–1875. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

