Risk-Taking Is Associated with Decreased Subjective Value Signals and Increased Prediction Error Signals in the Hot Columbia Card Task
- PMID: 38561225
- PMCID: PMC11112641
- DOI: 10.1523/JNEUROSCI.1337-23.2024
Risk-Taking Is Associated with Decreased Subjective Value Signals and Increased Prediction Error Signals in the Hot Columbia Card Task
Abstract
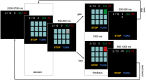
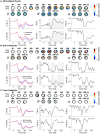
It remains a pressing concern to understand how neural computations relate to risky decisions. However, most observations of brain-behavior relationships in the risk-taking domain lack a rigorous computational basis or fail to emulate of the dynamic, sequential nature of real-life risky decision-making. Recent advances emphasize the role of neural prediction error (PE) signals. We modeled, according to prospect theory, the choices of n = 43 human participants (33 females, 10 males) performing an EEG version of the hot Columbia Card Task, featuring rounds of sequential decisions between stopping (safe option) and continuing with increasing odds of a high loss (risky option). Single-trial regression EEG analyses yielded a subjective value signal at centroparietal (300-700 ms) and frontocentral (>800 ms) electrodes and in the delta band, as well as PE signals tied to the feedback-related negativity, P3a, and P3b, and in the theta band. Higher risk preference (total number of risky choices) was linked to attenuated subjective value signals but increased PE signals. Higher P3-like activity associated with the most positive PE in each round predicted stopping in the present round but not risk-taking in the subsequent round. Our findings indicate that decreased representation of decision values and increased sensitivity to winning despite low odds (positive PE) facilitate risky choices at the subject level. Strong neural responses when gains are least expected (the most positive PE on each round) adaptively contribute to safer choices at the trial-by-trial level but do not affect risky choice at the round-by-round level.
Keywords: electroencephalography (EEG); expected utility; prediction error; prospect theory; sequential risk-taking.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188. 10.1214/aos/1013699998 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
