Transcriptional signature of durable effector T cells elicited by a replication defective HCMV vaccine
- PMID: 38561339
- PMCID: PMC10984989
- DOI: 10.1038/s41541-024-00860-w
Transcriptional signature of durable effector T cells elicited by a replication defective HCMV vaccine
Abstract
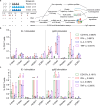
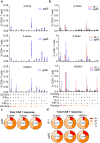
Human cytomegalovirus (HCMV) is a leading infectious cause of birth defects and the most common opportunistic infection that causes life-threatening diseases post-transplantation; however, an effective vaccine remains elusive. V160 is a live-attenuated replication defective HCMV vaccine that showed a 42.4% efficacy against primary HCMV infection among seronegative women in a phase 2b clinical trial. Here, we integrated the multicolor flow cytometry, longitudinal T cell receptor (TCR) sequencing, and single-cell RNA/TCR sequencing approaches to characterize the magnitude, phenotype, and functional quality of human T cell responses to V160. We demonstrated that V160 de novo induces IE-1 and pp65 specific durable polyfunctional effector CD8 T cells that are comparable to those induced by natural HCMV infection. We identified a variety of V160-responsive T cell clones which exhibit distinctive "transient" and "durable" expansion kinetics, and revealed a transcriptional signature that marks durable CD8 T cells post-vaccination. Our study enhances the understanding of human T-cell immune responses to V160 vaccination.
© 2024. The Author(s).
Conflict of interest statement
The study was in part funded by Merck & Co., which was the sponsor of the V160-002 clinical trial. The Sponsor had no influence on data collection and interpretation of the data. The authors declare no competing interests.
Figures




References
-
- Institute of Medicine. In Vaccines for the 21st Century: A Tool for Decision making (eds Stratton, K. R., Durch, J. S. & Lawrence, R. S.) (The National Academies Press, Washington, DC, 2000). 10.17226/5501. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

