Synthesis and biological evaluation of echinomycin analogues as potential colon cancer agent
- PMID: 38561454
- PMCID: PMC10985088
- DOI: 10.1038/s41598-024-58196-3
Synthesis and biological evaluation of echinomycin analogues as potential colon cancer agent
Abstract
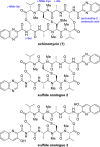
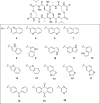
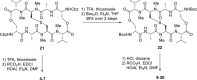
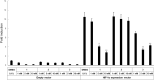
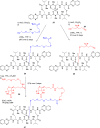
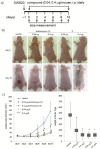
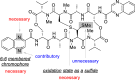
Colorectal cancer is the third most commonly diagnosed cancer and the second leading cause of cancer-related death, thus a novel chemotherapeutic agent for colon cancer therapy is needed. In this study, analogues of echinomycin, a cyclic peptide natural product with potent toxicity to several human cancer cell lines, were synthesized, and their biological activities against human colon cancer cells were investigated. Analogue 3 as well as 1 inhibit HIF-1α-mediated transcription. Notably, transcriptome analysis indicated that the cell cycle and its regulation were involved in the effects on cells treated with 3. Analogue 3 exhibited superior in vivo efficacy to echinomycin without significant toxicity in mouse xenograft model. The low dose of 3 needed to be efficacious in vivo is also noteworthy and our data suggest that 3 is an attractive and potentially novel agent for the treatment of colon cancer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Liposomal formulation of HIF-1α inhibitor echinomycin eliminates established metastases of triple-negative breast cancer.Nanomedicine. 2020 Oct;29:102278. doi: 10.1016/j.nano.2020.102278. Epub 2020 Jul 30. Nanomedicine. 2020. PMID: 32738299 Free PMC article.
-
Hypoxia inducible factor 1α expression and effects of its inhibitors in canine lymphoma.J Vet Med Sci. 2015 Nov;77(11):1405-12. doi: 10.1292/jvms.15-0258. Epub 2015 Jun 6. J Vet Med Sci. 2015. PMID: 26050843 Free PMC article.
-
Echinomycin mitigates ocular angiogenesis by transcriptional inhibition of the hypoxia-inducible factor-1.Exp Eye Res. 2021 May;206:108518. doi: 10.1016/j.exer.2021.108518. Epub 2021 Feb 25. Exp Eye Res. 2021. PMID: 33639134
-
HIF-1α inhibitor echinomycin reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect.J Transl Med. 2017 Feb 10;15(1):28. doi: 10.1186/s12967-017-1132-9. J Transl Med. 2017. PMID: 28183349 Free PMC article.
-
Inhibition of autophagy with chloroquine is effective in melanoma.J Surg Res. 2013 Sep;184(1):274-81. doi: 10.1016/j.jss.2013.04.055. Epub 2013 May 17. J Surg Res. 2013. PMID: 23706562
References
-
- WHO, Global Cancer Observation. https://gco.iarc.fr
-
- Corbaz R, Ettlinger L, Gaumann E, Keller-Schierlein W, Kradolfer F, Neipp L, Prelog V, Reusser P, Zahner H. Stoffwechselprodukte von actinomyceten. 7. Mitteilung. Echinomycin. Helv. Chim. Acta. 1957;40:199. doi: 10.1002/hlca.19570400124. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

