A serine-conjugated butyrate prodrug with high oral bioavailability suppresses autoimmune arthritis and neuroinflammation in mice
- PMID: 38561491
- PMCID: PMC11161413
- DOI: 10.1038/s41551-024-01190-x
A serine-conjugated butyrate prodrug with high oral bioavailability suppresses autoimmune arthritis and neuroinflammation in mice
Abstract
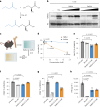
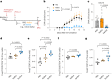
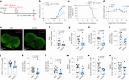
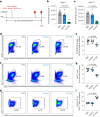
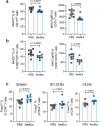
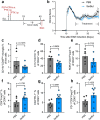
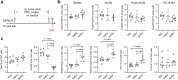
Butyrate-a metabolite produced by commensal bacteria-has been extensively studied for its immunomodulatory effects on immune cells, including regulatory T cells, macrophages and dendritic cells. However, the development of butyrate as a drug has been hindered by butyrate's poor oral bioavailability, owing to its rapid metabolism in the gut, its low potency (hence, necessitating high dosing), and its foul smell and taste. Here we report that the oral bioavailability of butyrate can be increased by esterifying it to serine, an amino acid transporter that aids the escape of the resulting odourless and tasteless prodrug (O-butyryl-L-serine, which we named SerBut) from the gut, enhancing its systemic uptake. In mice with collagen-antibody-induced arthritis (a model of rheumatoid arthritis) and with experimental autoimmune encephalomyelitis (a model of multiple sclerosis), we show that SerBut substantially ameliorated disease severity, modulated key immune cell populations systemically and in disease-associated tissues, and reduced inflammatory responses without compromising the global immune response to vaccination. SerBut may become a promising therapeutic for autoimmune and inflammatory diseases.
© 2024. The Author(s).
Conflict of interest statement
S.C., E.B., M.M.R., E.A.W. and J.A.H. are inventors on a patent application (WO2022226482A1) filed by the University of Chicago on uses of SerBut. The other authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

