Cyanobacterial Harmful Algal Mats (CyanoHAMs) in tropical rivers of central Mexico and their potential risks through toxin production
- PMID: 38561517
- PMCID: PMC10984904
- DOI: 10.1007/s10661-024-12568-4
Cyanobacterial Harmful Algal Mats (CyanoHAMs) in tropical rivers of central Mexico and their potential risks through toxin production
Abstract
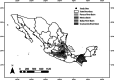
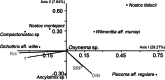
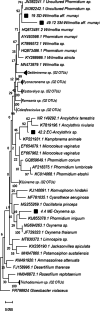
Cyanobacteria inhabiting lotic environments have been poorly studied and characterized in Mexico, despite their potential risks from cyanotoxin production. This article aims to fill this knowledge gap by assessing the importance of benthic cyanobacteria as potential cyanotoxin producers in central Mexican rivers through: (i) the taxonomic identification of cyanobacteria found in these rivers, (ii) the environmental characterization of their habitats, and (iii) testing for the presence of toxin producing genes in the encountered taxa. Additionally, we introduce and discuss the use of the term "CyanoHAMs" for lotic water environments. Populations of cyanobacteria were collected from ten mountain rivers and identified using molecular techniques. Subsequently, these taxa were evaluated for genes producing anatoxins and microcystins via PCR. Through RDA analyses, the collected cyanobacteria were grouped into one of three categories based on their environmental preferences for the following: (1) waters with high ionic concentrations, (2) cold-temperate waters, or (3) waters with high nutrient enrichment. Populations from six locations were identified to genus level: Ancylothrix sp., Cyanoplacoma sp., and Oxynema sp. The latter was found to contain the gene that produces anatoxins and microcystins in siliceous rivers, while Oxynema tested positive for the gene that produces microcystins in calcareous rivers. Our results suggest that eutrophic environments are not necessarily required for toxin-producing cyanobacteria. Our records of Compactonostoc, Oxynema, and Ancylothrix represent the first for Mexico. Four taxa were identified to species level: Wilmottia aff. murrayi, Nostoc tlalocii, Nostoc montejanii, and Dichothrix aff. willei, with only the first testing positive using PCR for anatoxin and microcystin-producing genes in siliceous rivers. Due to the differences between benthic growths with respect to planktonic ones, we propose the adoption of the term Cyanobacterial Harmful Algal Mats (CyanoHAMs) as a more precise descriptor for future studies.
Keywords: Anatoxins; Benthic cyanobacteria; Cyanobacterial Harmful Algal Mats; Microcystins; Polyphasic approach; River growths.
© 2024. The Author(s).
Conflict of interest statement
Not applicable
Figures





Similar articles
-
Widespread anatoxin-a detection in benthic cyanobacterial mats throughout a river network.PLoS One. 2018 May 18;13(5):e0197669. doi: 10.1371/journal.pone.0197669. eCollection 2018. PLoS One. 2018. PMID: 29775481 Free PMC article.
-
Multiple cyanotoxin congeners produced by sub-dominant cyanobacterial taxa in riverine cyanobacterial and algal mats.PLoS One. 2019 Dec 16;14(12):e0220422. doi: 10.1371/journal.pone.0220422. eCollection 2019. PLoS One. 2019. PMID: 31841562 Free PMC article.
-
Rise and fall of toxic benthic freshwater cyanobacteria (Anabaena spp.) in the Eel river: Buoyancy and dispersal.Harmful Algae. 2017 Jun;66:79-87. doi: 10.1016/j.hal.2017.05.007. Epub 2017 May 24. Harmful Algae. 2017. PMID: 28602256
-
Health and Environmental Impacts of Cyanobacteria and Cyanotoxins from Freshwater to Seawater.Toxins (Basel). 2025 Mar 7;17(3):126. doi: 10.3390/toxins17030126. Toxins (Basel). 2025. PMID: 40137899 Free PMC article. Review.
-
Emerging high throughput analyses of cyanobacterial toxins and toxic cyanobacteria.Adv Exp Med Biol. 2008;619:539-57. doi: 10.1007/978-0-387-75865-7_24. Adv Exp Med Biol. 2008. PMID: 18461783 Review.
Cited by
-
Changes in Microbial Community Assemblages Due To Urban Pollution, Detected via rRNA Gene Amplicon Sequencing in the Magdalena River, Mexico City.Microb Ecol. 2025 Aug 2;88(1):85. doi: 10.1007/s00248-025-02580-7. Microb Ecol. 2025. PMID: 40751837 Free PMC article.
References
-
- Aboal M, Puig M. Microcystin production in Rivularia colonies of calcareous streams from Mediterranean Spanish basins. Archiv Fur Hydrobiologie Supplements, Algological Studies. 2009;130:39–52.
-
- Aboal M, Puig MÁ, Asencio AD. Production of microcystins in calcareous Medi- terranean streams: The Alhárabe River, Segura River basin in south-east Spain. Journal of Applied Phycology. 2005;17:231–243. doi: 10.1007/s10811-005-2999-z. - DOI
-
- Almuhtaram H, Kibuye FA, Ajjampur S, Glover CM, Hofmann R, Gaget V, Zamyadi A, et al. State of knowledge on early warning tools for cyanobacteria detection. Ecological Indicators. 2021;133:108442. doi: 10.1016/j.ecolind.2021.108442. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

