A radiomics signature derived from CT imaging to predict MSI status and immunotherapy outcomes in gastric cancer: a multi-cohort study
- PMID: 38561648
- PMCID: PMC10985890
- DOI: 10.1186/s12885-024-12174-0
A radiomics signature derived from CT imaging to predict MSI status and immunotherapy outcomes in gastric cancer: a multi-cohort study
Abstract
Background: Accurate microsatellite instability (MSI) testing is essential for identifying gastric cancer (GC) patients eligible for immunotherapy. We aimed to develop and validate a CT-based radiomics signature to predict MSI and immunotherapy outcomes in GC.
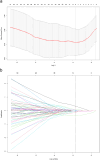
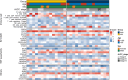
Methods: This retrospective multicohort study included a total of 457 GC patients from two independent medical centers in China and The Cancer Imaging Archive (TCIA) databases. The primary cohort (n = 201, center 1, 2017-2022), was used for signature development via Least Absolute Shrinkage and Selection Operator (LASSO) and logistic regression analysis. Two independent immunotherapy cohorts, one from center 1 (n = 184, 2018-2021) and another from center 2 (n = 43, 2020-2021), were utilized to assess the signature's association with immunotherapy response and survival. Diagnostic efficiency was evaluated using the area under the receiver operating characteristic curve (AUC), and survival outcomes were analyzed via the Kaplan-Meier method. The TCIA cohort (n = 29) was included to evaluate the immune infiltration landscape of the radiomics signature subgroups using both CT images and mRNA sequencing data.
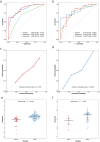
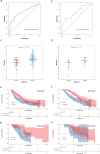
Results: Nine radiomics features were identified for signature development, exhibiting excellent discriminative performance in both the training (AUC: 0.851, 95%CI: 0.782, 0.919) and validation cohorts (AUC: 0.816, 95%CI: 0.706, 0.926). The radscore, calculated using the signature, demonstrated strong predictive abilities for objective response in immunotherapy cohorts (AUC: 0.734, 95%CI: 0.662, 0.806; AUC: 0.724, 95%CI: 0.572, 0.877). Additionally, the radscore showed a significant association with PFS and OS, with GC patients with a low radscore experiencing a significant survival benefit from immunotherapy. Immune infiltration analysis revealed significantly higher levels of CD8 + T cells, activated CD4 + B cells, and TNFRSF18 expression in the low radscore group, while the high radscore group exhibited higher levels of T cells regulatory and HHLA2 expression.
Conclusion: This study developed a robust radiomics signature with the potential to serve as a non-invasive biomarker for GC's MSI status and immunotherapy response, demonstrating notable links to post-immunotherapy PFS and OS. Additionally, distinct immune profiles were observed between low and high radscore groups, highlighting their potential clinical implications.
Keywords: Gastric cancer; Immunotherapy; MSI; Radiomics signature; mRNA-seq.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Development and external validation of a non-invasive imaging biomarker to estimate the microsatellite instability status of gastric cancer and its prognostic value: The combination of clinical and quantitative CT-imaging features.Eur J Radiol. 2023 May;162:110719. doi: 10.1016/j.ejrad.2023.110719. Epub 2023 Jan 27. Eur J Radiol. 2023. PMID: 36764010
-
Intratumoral and peritumoral CT-based radiomics for predicting the microsatellite instability in gastric cancer.Abdom Radiol (NY). 2024 May;49(5):1363-1375. doi: 10.1007/s00261-023-04165-9. Epub 2024 Feb 2. Abdom Radiol (NY). 2024. PMID: 38305796
-
Magnetic resonance-based radiomics nomogram for predicting microsatellite instability status in endometrial cancer.Quant Imaging Med Surg. 2023 Jan 1;13(1):108-120. doi: 10.21037/qims-22-255. Epub 2022 Oct 19. Quant Imaging Med Surg. 2023. PMID: 36620141 Free PMC article.
-
Radiomics analysis of contrast-enhanced CT predicts lymphovascular invasion and disease outcome in gastric cancer: a preliminary study.Cancer Imaging. 2020 Apr 5;20(1):24. doi: 10.1186/s40644-020-00302-5. Cancer Imaging. 2020. PMID: 32248822 Free PMC article.
-
Preoperative radiomics models using CT and MRI for microsatellite instability in colorectal cancer: a systematic review and meta-analysis.Abdom Radiol (NY). 2025 May 10. doi: 10.1007/s00261-025-04981-1. Online ahead of print. Abdom Radiol (NY). 2025. PMID: 40347255 Review.
Cited by
-
Gastrointestinal tumor personalized immunotherapy: an integrated analysis from molecular genetics to imaging biomarkers.Therap Adv Gastroenterol. 2025 Apr 23;18:17562848251333527. doi: 10.1177/17562848251333527. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40297204 Free PMC article. Review.
-
From Images to Genes: Radiogenomics Based on Artificial Intelligence to Achieve Non-Invasive Precision Medicine in Cancer Patients.Adv Sci (Weinh). 2025 Jan;12(2):e2408069. doi: 10.1002/advs.202408069. Epub 2024 Nov 13. Adv Sci (Weinh). 2025. PMID: 39535476 Free PMC article. Review.
References
-
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75. doi: 10.1016/S0140-6736(17)33326-3. - DOI - PMC - PubMed
-
- Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, et al. Effect of Neoadjuvant Chemotherapy followed by Surgical Resection on Survival in patients with Limited Metastatic gastric or gastroesophageal Junction Cancer: the AIO-FLOT3 Trial. JAMA Oncol. 2017;3(9):1237–44. doi: 10.1001/jamaoncol.2017.0515. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

