Transcriptional dynamics during Rhodococcus erythropolis infection with phage WC1
- PMID: 38561651
- PMCID: PMC10986025
- DOI: 10.1186/s12866-024-03241-4
Transcriptional dynamics during Rhodococcus erythropolis infection with phage WC1
Abstract
Background: Belonging to the Actinobacteria phylum, members of the Rhodococcus genus thrive in soil, water, and even intracellularly. While most species are non-pathogenic, several cause respiratory disease in animals and, more rarely, in humans. Over 100 phages that infect Rhodococcus species have been isolated but despite their importance for Rhodococcus ecology and biotechnology applications, little is known regarding the molecular genetic interactions between phage and host during infection. To address this need, we report RNA-Seq analysis of a novel Rhodococcus erythopolis phage, WC1, analyzing both the phage and host transcriptome at various stages throughout the infection process.
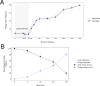
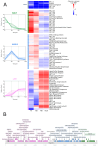
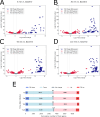
Results: By five minutes post-infection WC1 showed upregulation of a CAS-4 family exonuclease, putative immunity repressor, an anti-restriction protein, while the host showed strong upregulation of DNA replication, SOS repair, and ribosomal protein genes. By 30 min post-infection, WC1 DNA synthesis genes were strongly upregulated while the host showed increased expression of transcriptional and translational machinery and downregulation of genes involved in carbon, energy, and lipid metabolism pathways. By 60 min WC1 strongly upregulated structural genes while the host showed a dramatic disruption of metal ion homeostasis. There was significant expression of both host and phage non-coding genes at all time points. While host gene expression declined over the course of infection, our results indicate that phage may exert more selective control, preserving the host's regulatory mechanisms to create an environment conducive for virion production.
Conclusions: The Rhodococcus genus is well recognized for its ability to synthesize valuable compounds, particularly steroids, as well as its capacity to degrade a wide range of harmful environmental pollutants. A detailed understanding of these phage-host interactions and gene expression is not only essential for understanding the ecology of this important genus, but will also facilitate development of phage-mediated strategies for bioremediation as well as biocontrol in industrial processes and biomedical applications. Given the current lack of detailed global gene expression studies on any Rhodococcus species, our study addresses a pressing need to identify tools and genes, such as F6 and rpf, that can enhance the capacity of Rhodococcus species for bioremediation, biosynthesis and pathogen control.
Keywords: Rhodococcus; Phage; RNA-Seq; Transcriptome; WC1.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Characterization of the genome of the polyvalent lytic bacteriophage GTE2, which has potential for biocontrol of Gordonia-, Rhodococcus-, and Nocardia-stabilized foams in activated sludge plants.Appl Environ Microbiol. 2011 Jun;77(12):3923-9. doi: 10.1128/AEM.00025-11. Epub 2011 Apr 15. Appl Environ Microbiol. 2011. PMID: 21498753 Free PMC article.
-
Ribosome profiling reveals downregulation of UMP biosynthesis as the major early response to phage infection.Microbiol Spectr. 2024 Apr 2;12(4):e0398923. doi: 10.1128/spectrum.03989-23. Epub 2024 Mar 7. Microbiol Spectr. 2024. PMID: 38451091 Free PMC article.
-
Isolation and characterization of soilborne virulent bacteriophages infecting the pathogen Rhodococcus equi.J Appl Microbiol. 2013 Jun;114(6):1625-33. doi: 10.1111/jam.12194. Epub 2013 Apr 5. J Appl Microbiol. 2013. PMID: 23495898
-
The biology and genetics of the genus Rhodococcus.Annu Rev Microbiol. 1992;46:193-218. doi: 10.1146/annurev.mi.46.100192.001205. Annu Rev Microbiol. 1992. PMID: 1444254 Review.
-
Rhodococcus: A promising genus of actinomycetes for the bioremediation of organic and inorganic contaminants.J Environ Manage. 2022 Dec 1;323:116220. doi: 10.1016/j.jenvman.2022.116220. Epub 2022 Sep 15. J Environ Manage. 2022. PMID: 36116255 Review.
References
-
- Kim D, Choi KY, Yoo M, Zylstra GJ, Kim E. Biotechnol Potential Rhodococcus Biodegradative Pathways. 2018;28:1037–51. - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

