A hypervirulent Acinetobacter baumannii strain has robust anti-phagocytosis ability
- PMID: 38561652
- PMCID: PMC10983618
- DOI: 10.1186/s12866-024-03264-x
A hypervirulent Acinetobacter baumannii strain has robust anti-phagocytosis ability
Abstract
Background: Acinetobacter baumannii (A. baumannii) is associated with both hospital-acquired infections (HAP) and community-acquired pneumonia (CAP). In this study, we present a novel CAP-associated A. baumannii (CAP-AB) strain causing severe pneumonia in an afore healthy male patient without underlying conditions. Subsequently, we investigated the pathogenicity and immunogenicity of this CAP-AB strain using a mice pneumonia model.
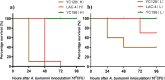
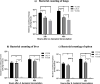
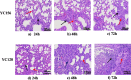
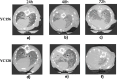
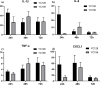
Results: A 58-year-old male patient with no underlying conditions experienced worsening symptoms of a productive cough, sputum, and fever that developed acutely, in just 24 h. The diagnosis was severe community-acquired pneumonia (CAP) and type-1 respiratory failure. An A. baumannii strain was isolated from his sputum and blood cultures. To gain a deeper understanding of the rapid progression of its pathology, we utilized the CAP-associated A. baumannii strain YC128, a previously obtained hospital-acquired pneumonia A. baumannii (HAP-AB) strain YC156, and a highly virulent A. baumannii control strain LAC-4 to construct a mouse pneumonia model, and subsequently compared the mortality rate of the three groups. Following inoculation with 107 CFU of A. baumannii, the mortality rate for the YC128, LAC-4, and YC156 groups was 60% (6/10), 30% (3/10), and 0%, respectively. The bacterial burden within the pulmonary, liver, and spleen tissues of mice in the YC128 group was significantly higher than that of the YC156 group, and slightly higher than that of the LAC-4 group. Pathological analysis of lung tissue using HE-staining revealed that the inflammatory pathological changes in mice from the YC128 group were significantly more severe than those in the YC156 group. Additionally, CT scan images displayed more pronounced inflammation in the lungs of mice from the YC128 group compared to the YC156 group. Local levels of cytokines/chemokines such as IL-1β, IL-6, TNF-α, and CXCL1 were assessed via RT-qPCR in lung tissues. In comparison with the YC156 strain, the highly virulent YC128 strain induced the expression of proinflammatory cytokines more rapidly and severely. Furthermore, we examined the in vitro anti-phagocytosis ability of YC128 and YC156 strains against mice peritoneal macrophages, revealing that the highly virulent YC128 isolate displayed greater resistance to macrophage uptake in contrast to YC156. Results from Whole Genome Sequencing (WGS) indicated that YC128 harbored a complete type VI secretion system (T6SS) gene cluster, while YC156 lacked the majority of genes within the T6SS gene cluster. The other virulence-related genes exhibited minimal differences between YC128 and YC156. Drawing from previous studies, we postulated that the T6SS is linked to the hypervirulence and robust anti-phagocytic ability of YC128.
Conclusions: This article reports on the isolation of a novel hypervirulent CAP-AB strain, YC128, from a severe CAP patient. The results demonstrate that this CAP-AB strain, YC128, is capable of inducing fatal pneumonia and extrapulmonary dissemination in a mouse pneumonia model. Moreover, this highly virulent CAP-AB strain exhibits significantly stronger anti-phagocytic abilities compared to the HAP-AB YC156 strain. Genome sequencing comparisons reveal that the heightened hypervirulence and enhanced anti-phagocytosis abilities observed in YC128 may be attributed to the presence of the T6SS.
Keywords: Acinetobacter baumannii; Anti-phagocytosis; Community-acquired pneumonia; Hypervirulent; Type VI secretion system.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A case-control study of community-acquired Acinetobacter baumannii pneumonia and melioidosis pneumonia in northeast Thailand: an emerging fatal disease with unique clinical features.Diagn Microbiol Infect Dis. 2017 Jan;87(1):79-86. doi: 10.1016/j.diagmicrobio.2016.10.014. Epub 2016 Oct 11. Diagn Microbiol Infect Dis. 2017. PMID: 27789057
-
A mouse model of Acinetobacter baumannii-associated pneumonia using a clinically isolated hypervirulent strain.Antimicrob Agents Chemother. 2013 Aug;57(8):3601-13. doi: 10.1128/AAC.00944-13. Epub 2013 May 20. Antimicrob Agents Chemother. 2013. PMID: 23689726 Free PMC article.
-
Diagnosis of severe community-acquired pneumonia caused by Acinetobacter baumannii through next-generation sequencing: a case report.BMC Infect Dis. 2020 Jan 15;20(1):45. doi: 10.1186/s12879-019-4733-5. BMC Infect Dis. 2020. PMID: 31941459 Free PMC article.
-
Antibiotic resistance in community-acquired pneumonia caused by Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, and Acinetobacter baumannii.Chest. 2009 Oct;136(4):1119-1127. doi: 10.1378/chest.09-0285. Chest. 2009. PMID: 19809053 Review.
-
Urinary tract colonization is enhanced by a plasmid that regulates uropathogenic Acinetobacter baumannii chromosomal genes.Nat Commun. 2019 Jun 24;10(1):2763. doi: 10.1038/s41467-019-10706-y. Nat Commun. 2019. PMID: 31235751 Free PMC article.
References
-
- Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Guidance on the Treatment of AmpC beta-Lactamase-Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin Infect Dis. 2022;74(12):2089–2114. doi: 10.1093/cid/ciab1013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

