Acyl-CoA-binding protein (ACBP) genes involvement in response to abiotic stress and exogenous hormone application in barley (Hordeum vulgare L.)
- PMID: 38561660
- PMCID: PMC10985865
- DOI: 10.1186/s12870-024-04944-6
Acyl-CoA-binding protein (ACBP) genes involvement in response to abiotic stress and exogenous hormone application in barley (Hordeum vulgare L.)
Abstract
Background: Acyl-CoA-Binding proteins (ACBPs) function as coenzyme A transporters and play important roles in regulating plant growth and development in response to abiotic stress and phytohormones, as well as in membrane repair. To date, the ACBP family has not been a comprehensively characterized in barley (Hordeum vulgare L.).
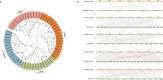
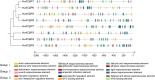
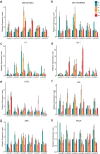
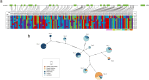
Results: Eight ACBP genes were identified in the barley genome and named as HvACBP1-8. The analysis of the proteins structure and promoter elements of HvACBP suggested its potential functions in plant growth, development, and stress response. These HvACBPs are expressed in specific tissues and organs following induction by abiotic stressors such as drought, salinity, UV-B exposure, temperature extremes, and exposure to exogenous phytohormones. The HvACBP7 and HvACBP8 amino acid sequences were conserved during the domestication of Tibetan Qingke barley.
Conclusions: Acyl-CoA-binding proteins may play important roles in barley growth and environmental adaptation. This study provides foundation for further analyses of the biological functions of HvACBPs in the barley stress response.
Keywords: Acyl-CoA-binding protein (ACBP); Barley stress response; Gene expression pattern.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Genome-wide identification and expression profile under abiotic stress of the barley non-specific lipid transfer protein gene family and its Qingke Orthologues.BMC Genomics. 2021 Sep 20;22(1):674. doi: 10.1186/s12864-021-07958-8. BMC Genomics. 2021. PMID: 34544387 Free PMC article.
-
Molecular characterization, expression and functional analysis of acyl-CoA-binding protein gene family in maize (Zea mays).BMC Plant Biol. 2021 Feb 15;21(1):94. doi: 10.1186/s12870-021-02863-4. BMC Plant Biol. 2021. PMID: 33588749 Free PMC article.
-
Molecular Characterization of the Acyl-CoA-Binding Protein Genes Reveals Their Significant Roles in Oil Accumulation and Abiotic Stress Response in Cotton.Genes (Basel). 2023 Apr 1;14(4):859. doi: 10.3390/genes14040859. Genes (Basel). 2023. PMID: 37107617 Free PMC article.
-
New roles for acyl-CoA-binding proteins (ACBPs) in plant development, stress responses and lipid metabolism.Prog Lipid Res. 2011 Apr;50(2):141-51. doi: 10.1016/j.plipres.2010.11.002. Epub 2010 Dec 7. Prog Lipid Res. 2011. PMID: 21144863 Review.
-
Plant Acyl-CoA-Binding Proteins-Their Lipid and Protein Interactors in Abiotic and Biotic Stresses.Cells. 2021 Apr 30;10(5):1064. doi: 10.3390/cells10051064. Cells. 2021. PMID: 33946260 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 2023CYJSTX03-19/Modern Agro-Industry Technology Research System of Shanxi Province, China
- 2023CYJSTX03-19/Modern Agro-Industry Technology Research System of Shanxi Province, China
- 2023CYJSTX03-19/Modern Agro-Industry Technology Research System of Shanxi Province, China
- 2023CYJSTX03-19/Modern Agro-Industry Technology Research System of Shanxi Province, China
- 2023CYJSTX03-19/Modern Agro-Industry Technology Research System of Shanxi Province, China
- 202204010910001-06/National Laboratory for Minor Crops Germplasm Innovation and Molecular Breeding, China (in preparation)
- 202204010910001-06/National Laboratory for Minor Crops Germplasm Innovation and Molecular Breeding, China (in preparation)
- 202204010910001-06/National Laboratory for Minor Crops Germplasm Innovation and Molecular Breeding, China (in preparation)
- 202204010910001-06/National Laboratory for Minor Crops Germplasm Innovation and Molecular Breeding, China (in preparation)
- 202204010910001-06/National Laboratory for Minor Crops Germplasm Innovation and Molecular Breeding, China (in preparation)
- 202204010910001-06/National Laboratory for Minor Crops Germplasm Innovation and Molecular Breeding, China (in preparation)
- 202204010910001-06/National Laboratory for Minor Crops Germplasm Innovation and Molecular Breeding, China (in preparation)
- CARS-05/China Agriculture Research System of MOF and MORA
- CARS-05/China Agriculture Research System of MOF and MORA
- CARS-05/China Agriculture Research System of MOF and MORA
- CARS-05/China Agriculture Research System of MOF and MORA
LinkOut - more resources
Full Text Sources

