A randomized feasibility trial of medium chain triglyceride-supplemented ketogenic diet in people with Parkinson's disease
- PMID: 38561682
- PMCID: PMC10983636
- DOI: 10.1186/s12883-024-03603-5
A randomized feasibility trial of medium chain triglyceride-supplemented ketogenic diet in people with Parkinson's disease
Abstract
Background: A ketogenic diet (KD) may benefit people with neurodegenerative disorders marked by mitochondrial depolarization/insufficiency, including Parkinson's disease (PD).
Objective: Evaluate whether a KD supplemented by medium chain triglyceride (MCT-KD) oil is feasible and acceptable for PD patients. Furthermore, we explored the effects of MCT-KD on blood ketone levels, metabolic parameters, levodopa absorption, mobility, nonmotor symptoms, simple motor and cognitive tests, autonomic function, and resting-state electroencephalography (rsEEG).
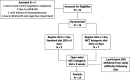
Methods: A one-week in-hospital, double-blind, randomized, placebo-controlled diet (MCT-KD vs. standard diet (SD)), followed by an at-home two-week open-label extension. The primary outcome was KD feasibility and acceptability. The secondary outcome was the change in Timed Up & Go (TUG) on day 7 of the diet intervention. Additional exploratory outcomes included the N-Back task, Unified Parkinson's Disease Rating Scale, Non-Motor Symptom Scale, and rsEEG connectivity.
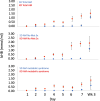
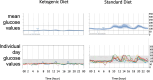
Results: A total of 15/16 subjects completed the study. The mean acceptability was 2.3/3, indicating willingness to continue the KD. Day 7 TUG time was not significantly different between the SD and KD groups. The nonmotor symptom severity score was reduced at the week 3 visit and to a greater extent in the KD group. UPDRS, 3-back, and rsEEG measures were not significantly different between groups. Blood ketosis was attained by day 4 in the KD group and to a greater extent at week 3 than in the SD group. The plasma levodopa metabolites DOPAC and dopamine both showed nonsignificant increasing trends over 3 days in the KD vs. SD groups.
Conclusions: An MCT-supplemented KD is feasible and acceptable to PD patients but requires further study to understand its effects on symptoms and disease.
Trial registration: Trial Registration Number NCT04584346, registration dates were Oct 14, 2020 - Sept 13, 2022.
Keywords: Biomarker; Clinical trial; Ketogenic diet; Ketosis; Parkinson’s disease; Pilot study.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

