Significance of hepatitis B virus capsid dephosphorylation via polymerase
- PMID: 38561844
- PMCID: PMC10983652
- DOI: 10.1186/s12929-024-01022-9
Significance of hepatitis B virus capsid dephosphorylation via polymerase
Abstract
Background: It is generally believed that hepatitis B virus (HBV) core protein (HBc) dephosphorylation (de-P) is important for viral DNA synthesis and virion secretion. HBV polymerase contains four domains for terminal protein, spacer, reverse transcriptase, and RNase H activities.
Methods: HBV Polymerase mutants were transfected into HuH-7 cells and assayed for replication and HBc de-P by the Phos-tag gel analysis. Infection assay was performed by using a HepG2-NTCP-AS2 cell line.
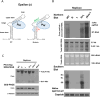
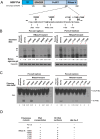
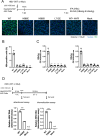
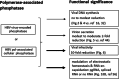
Results: Here, we show that a novel phosphatase activity responsible for HBc de-P can be mapped to the C-terminal domain of the polymerase overlapping with the RNase H domain. Surprisingly, while HBc de-P is crucial for viral infectivity, it is essential for neither viral DNA synthesis nor virion secretion. The potential origin, significance, and mechanism of this polymerase-associated phosphatase activity are discussed in the context of an electrostatic homeostasis model. The Phos-tag gel analysis revealed an intriguing pattern of "bipolar distribution" of phosphorylated HBc and a de-P HBc doublet.
Conclusions: It remains unknown if such a polymerase-associated phosphatase activity can be found in other related biosystems. This polymerase-associated phosphatase activity could be a druggable target in clinical therapy for hepatitis B.
Keywords: Capsids dephosphorylation (de-P); HBV core protein (HBc); Hepatitis B virus (HBV); Phosphatase; Polymerase (pol); RNase H domain.
© 2024. The Author(s).
Conflict of interest statement
We declare no competing financial or non-financial interests.
Figures



Similar articles
-
Capsid Phosphorylation State and Hepadnavirus Virion Secretion.J Virol. 2017 Apr 13;91(9):e00092-17. doi: 10.1128/JVI.00092-17. Print 2017 May 1. J Virol. 2017. PMID: 28228589 Free PMC article.
-
Cell-Free Hepatitis B Virus Capsid Assembly Dependent on the Core Protein C-Terminal Domain and Regulated by Phosphorylation.J Virol. 2016 May 27;90(12):5830-5844. doi: 10.1128/JVI.00394-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27076641 Free PMC article.
-
Common and Distinct Capsid and Surface Protein Requirements for Secretion of Complete and Genome-Free Hepatitis B Virions.J Virol. 2018 Jun 29;92(14):e00272-18. doi: 10.1128/JVI.00272-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743374 Free PMC article.
-
Hepatitis B Virus Capsid: The Core in Productive Entry and Covalently Closed Circular DNA Formation.Viruses. 2023 Feb 28;15(3):642. doi: 10.3390/v15030642. Viruses. 2023. PMID: 36992351 Free PMC article. Review.
-
Phosphorylation of the Arginine-Rich C-Terminal Domains of the Hepatitis B Virus (HBV) Core Protein as a Fine Regulator of the Interaction between HBc and Nucleic Acid.Viruses. 2020 Jul 8;12(7):738. doi: 10.3390/v12070738. Viruses. 2020. PMID: 32650547 Free PMC article. Review.
Cited by
-
From the Cytoplasm into the Nucleus-Hepatitis B Virus Travel and Genome Repair.Microorganisms. 2025 Jan 14;13(1):157. doi: 10.3390/microorganisms13010157. Microorganisms. 2025. PMID: 39858925 Free PMC article. Review.
-
HBV polymerase recruits the phosphatase PP1 to dephosphorylate HBc-Ser170 to complete encapsidation.PLoS Pathog. 2025 Feb 11;21(2):e1012905. doi: 10.1371/journal.ppat.1012905. eCollection 2025 Feb. PLoS Pathog. 2025. PMID: 39932960 Free PMC article.
-
Epitranscriptomic cytidine methylation of the hepatitis B viral RNA is essential for viral reverse transcription and particle production.Proc Natl Acad Sci U S A. 2024 Jun 11;121(24):e2400378121. doi: 10.1073/pnas.2400378121. Epub 2024 Jun 3. Proc Natl Acad Sci U S A. 2024. PMID: 38830096 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

