Effect of liraglutide on thigh muscle fat and muscle composition in adults with overweight or obesity: Results from a randomized clinical trial
- PMID: 38561962
- PMCID: PMC11154779
- DOI: 10.1002/jcsm.13445
Effect of liraglutide on thigh muscle fat and muscle composition in adults with overweight or obesity: Results from a randomized clinical trial
Abstract
Background: Excess muscle fat is observed in obesity and associated with greater burden of cardiovascular risk factors and higher risk of mortality. Liraglutide reduces total body weight and visceral fat but its effect on muscle fat and adverse muscle composition is unknown.
Methods: This is a pre-specified secondary analysis of a randomized, double-blind, placebo-controlled trial that examined the effects of liraglutide plus a lifestyle intervention on visceral adipose tissue and ectopic fat among adults without diabetes with body mass index ≥30 kg/m2 or ≥27 kg/m2 and metabolic syndrome. Participants were randomly assigned to a once-daily subcutaneous injection of liraglutide (target dose 3.0 mg) or matching placebo for 40 weeks. Body fat distribution and muscle composition was assessed by magnetic resonance imaging at baseline and 40-week follow-up. Muscle composition was described by the combination of thigh muscle fat and muscle volume. Treatment difference (95% confidence intervals [CI]) was calculated by least-square means adjusted for baseline thigh muscle fat. The association between changes in thigh muscle fat and changes in body weight were assessed using Spearman correlation coefficients. The effect of liraglutide versus placebo on adverse muscle composition, denoted by high thigh muscle fat and low thigh muscle volume, was explored.
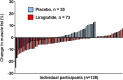
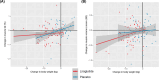
Results: Among the 128 participants with follow-up imaging (92.2% women, 36.7% Black), median muscle fat at baseline was 7.8%. The mean percent change in thigh muscle fat over median follow-up of 36 weeks was -2.87% among participants randomized to liraglutide (n = 73) and 0.05% in the placebo group (absolute change: -0.23% vs. 0.01%). The estimated treatment difference adjusted for baseline thigh muscle fat was -0.24% (95% CI, -0.41 to -0.06, P-value 0.009). Longitudinal change in thigh muscle fat was significantly associated with change in body weight in the placebo group but not the liraglutide group. The proportion of participants with adverse muscle composition decreased from 11.0% to 8.2% over follow-up with liraglutide, but there was no change with placebo.
Conclusions: In a cohort of predominantly women with overweight or obesity in the absence of diabetes, once-daily subcutaneous liraglutide was associated with a reduction in thigh muscle fat and adverse muscle composition compared with placebo. The contribution of muscle fat improvement to the cardiometabolic benefits of liraglutide requires further study.
Keywords: Body composition; Liraglutide; Muscle fat; Muscle volume; Obesity; Overweight.
© 2024 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
Dr. Pandey is supported by the Texas Health Resources Clinical Scholarship, the Gilead Sciences Research Scholar Program, the National Institute of Aging GEMSSTAR Grant (1R03AG067960‐01), and Applied Therapeutics; has served on the advisory board for Roche Diagnostics; and has received nonfinancial support from Pfizer and Merck. Dr. Patel has served as a consultant to Novo Nordisk. Ms. Linge and Dr. Leinhard are employees and stockholders of AMRA Medical AB. Dr. Anker reports grants and personal fees from Vifor Int. and Abbott Vascular, and personal fees from Astra‐Zeneca, Bayer, Brahms, Boehringer Ingelheim, Cardiac Dimensions, Novartis, Occlutech, Servier, and Vifor Int. Dr. Butler has served as a consultant for Abbott, Adrenomed, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, CVRx, G3 Pharmaceutical, Innolife, Janssen, LivaNova, Medtronic, Merck, Novartis, Novo Nordisk, Occlutech, Relypsa, Roche, and Vifor. Dr. Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and reports receiving research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, Sun Pharma, and the Toronto Knowledge Translation Working Group. He is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not‐for‐profit physician organization. Dr. Joshi has received Grant support from Novo Nordisk, as well as Amgen and Novartis. Consulting Novartis. Equity G3 Therapeutics. Dr. Neeland has received speaker and consultancy fees from Boehringer Ingelheim/Lilly Alliance, Merck, Nestle Health Sciences, and AMRA Medical; and grant support from Novo Nordisk.
Figures

References
-
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017‐2018. NCHS Data Brief 2020;1–8. - PubMed

