A reference-grade genome of the xerophyte Ammopiptanthus mongolicus sheds light on its evolution history in legumes and drought-tolerance mechanisms
- PMID: 38561965
- PMCID: PMC11287142
- DOI: 10.1016/j.xplc.2024.100891
A reference-grade genome of the xerophyte Ammopiptanthus mongolicus sheds light on its evolution history in legumes and drought-tolerance mechanisms
Abstract
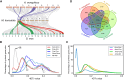
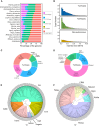
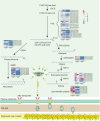
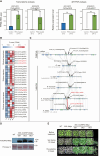
Plants that grow in extreme environments represent unique sources of stress-resistance genes and mechanisms. Ammopiptanthus mongolicus (Leguminosae) is a xerophytic evergreen broadleaf shrub native to semi-arid and desert regions; however, its drought-tolerance mechanisms remain poorly understood. Here, we report the assembly of a reference-grade genome for A. mongolicus, describe its evolutionary history within the legume family, and examine its drought-tolerance mechanisms. The assembled genome is 843.07 Mb in length, with 98.7% of the sequences successfully anchored to the nine chromosomes of A. mongolicus. The genome is predicted to contain 47 611 protein-coding genes, and 70.71% of the genome is composed of repetitive sequences; these are dominated by transposable elements, particularly long-terminal-repeat retrotransposons. Evolutionary analyses revealed two whole-genome duplication (WGD) events at 130 and 58 million years ago (mya) that are shared by the genus Ammopiptanthus and other legumes, but no species-specific WGDs were found within this genus. Ancestral genome reconstruction revealed that the A. mongolicus genome has undergone fewer rearrangements than other genomes in the legume family, confirming its status as a "relict plant". Transcriptomic analyses demonstrated that genes involved in cuticular wax biosynthesis and transport are highly expressed, both under normal conditions and in response to polyethylene glycol-induced dehydration. Significant induction of genes related to ethylene biosynthesis and signaling was also observed in leaves under dehydration stress, suggesting that enhanced ethylene response and formation of thick waxy cuticles are two major mechanisms of drought tolerance in A. mongolicus. Ectopic expression of AmERF2, an ethylene response factor unique to A. mongolicus, can markedly increase the drought tolerance of transgenic Arabidopsis thaliana plants, demonstrating the potential for application of A. mongolicus genes in crop improvement.
Keywords: Ammopiptanthus mongolicus; cuticular wax; drought tolerance; ethylene; genome evolution; genome sequencing.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Comparative transcriptome profiling of a desert evergreen shrub, Ammopiptanthus mongolicus, in response to drought and cold stresses.BMC Genomics. 2014 Aug 9;15(1):671. doi: 10.1186/1471-2164-15-671. BMC Genomics. 2014. PMID: 25108399 Free PMC article.
-
Identification of stress-responsive genes in Ammopiptanthus mongolicus using ESTs generated from cold- and drought-stressed seedlings.BMC Plant Biol. 2013 Jun 5;13:88. doi: 10.1186/1471-2229-13-88. BMC Plant Biol. 2013. PMID: 23734749 Free PMC article.
-
De novo sequencing and analysis of root transcriptome using 454 pyrosequencing to discover putative genes associated with drought tolerance in Ammopiptanthus mongolicus.BMC Genomics. 2012 Jun 21;13:266. doi: 10.1186/1471-2164-13-266. BMC Genomics. 2012. PMID: 22721448 Free PMC article.
-
Characterization of the Transcriptome of the Xerophyte Ammopiptanthus mongolicus Leaves under Drought Stress by 454 Pyrosequencing.PLoS One. 2015 Aug 27;10(8):e0136495. doi: 10.1371/journal.pone.0136495. eCollection 2015. PLoS One. 2015. PMID: 26313687 Free PMC article.
-
MdCER2 conferred to wax accumulation and increased drought tolerance in plants.Plant Physiol Biochem. 2020 Apr;149:277-285. doi: 10.1016/j.plaphy.2020.02.013. Epub 2020 Feb 14. Plant Physiol Biochem. 2020. PMID: 32088579 Review.
Cited by
-
Ammopiptanthus nanus Population Dynamics: Bridging the Gap Between Genetic Variation and Ecological Distribution Patterns.Biology (Basel). 2025 Jan 21;14(2):105. doi: 10.3390/biology14020105. Biology (Basel). 2025. PMID: 40001873 Free PMC article.
-
The chromosome-scale genome of black wolfberry (Lycium ruthenicum) provides useful genomic resources for identifying genes related to anthocyanin biosynthesis and disease resistance.Plant Divers. 2025 Jan 6;47(2):201-213. doi: 10.1016/j.pld.2025.01.001. eCollection 2025 Mar. Plant Divers. 2025. PMID: 40182488 Free PMC article.
-
Response of different cotton genotypes to salt stress and re-watering.BMC Plant Biol. 2025 May 5;25(1):587. doi: 10.1186/s12870-025-06534-6. BMC Plant Biol. 2025. PMID: 40320527 Free PMC article.
-
Comparative transcriptome and coexpression network analysis revealed the regulatory mechanism of Astragalus cicer L. in response to salt stress.BMC Plant Biol. 2024 Aug 30;24(1):817. doi: 10.1186/s12870-024-05531-5. BMC Plant Biol. 2024. PMID: 39210248 Free PMC article.
-
LGRPv2: A high-value platform for the advancement of Fabaceae genomics.Plant Biotechnol J. 2025 Sep;23(9):4057-4075. doi: 10.1111/pbi.70220. Epub 2025 Jun 22. Plant Biotechnol J. 2025. PMID: 40545607 Free PMC article.
References
-
- Bruneau A., Doyle J.J., Herendeen P., Hughes C., Kenicer G., Lewis G., Mackinder B., Pennington R.T., Sanderson M.J., Wojciechowski M.F., et al. Legume phylogeny and classification in the 21st century: Progress, prospects and lessons for other species-rich clades. Taxon. 2013;62:217–248. doi: 10.12705/622.8. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

