Protective effects of amphetamine and methylphenidate against dopaminergic neurotoxicants in SH-SY5Y cells
- PMID: 38562456
- PMCID: PMC10982568
- DOI: 10.1016/j.crtox.2024.100165
Protective effects of amphetamine and methylphenidate against dopaminergic neurotoxicants in SH-SY5Y cells
Abstract
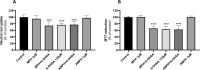
Full treatment of the second most common neurodegenerative disorder, Parkinson's disease (PD), is still considered an unmet need. As the psychostimulants, amphetamine (AMPH) and methylphenidate (MPH), were shown to be neuroprotective against stroke and other neuronal injury diseases, this study aimed to evaluate their neuroprotective potential against two dopaminergic neurotoxicants, 6-hydroxydopamine (6-OHDA) and paraquat (PQ), in differentiated human dopaminergic SH-SY5Y cells. Neither cytotoxicity nor mitochondrial membrane potential changes were seen following a 24-hour exposure to either therapeutic concentration of AMPH or MPH (0.001-10 μM). On the other hand, a 24-hour exposure to 6-OHDA (31.25-500 μM) or PQ (100-5000 μM) induced concentration-dependent mitochondrial dysfunction, assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, and lysosomal damage, evaluated by the neutral red uptake assay. The lethal concentrations 25 and 50 retrieved from the concentration-toxicity curves in the MTT assay were 99.9 µM and 133.6 µM for 6-OHDA, or 422 µM and 585.8 µM for PQ. Both toxicants caused mitochondrial membrane potential depolarization, but only 6-OHDA increased reactive oxygen species (ROS). Most importantly, PQ-induced toxicity was partially prevented by 1 μM of AMPH or MPH. Nonetheless, neither AMPH nor MPH could prevent 6-OHDA toxicity in this experimental model. According to these findings, AMPH and MPH may provide some neuroprotection against PQ-induced neurotoxicity, but further investigation is required to determine the exact mechanism underlying this protection.
Keywords: 6-Hydroxydopamine; Amphetamine; Methylphenidate; Neuroprotection; Paraquat; SH-SY5Y cells.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Toxicity of the amphetamine metabolites 4-hydroxyamphetamine and 4-hydroxynorephedrine in human dopaminergic differentiated SH-SY5Y cells.Toxicol Lett. 2017 Mar 5;269:65-76. doi: 10.1016/j.toxlet.2017.01.012. Epub 2017 Jan 20. Toxicol Lett. 2017. PMID: 28115274
-
Phytohormone Abscisic Acid Protects Human Neuroblastoma SH-SY5Y Cells Against 6-Hydroxydopamine-Induced Neurotoxicity Through Its Antioxidant and Antiapoptotic Properties.Rejuvenation Res. 2019 Apr;22(2):99-108. doi: 10.1089/rej.2018.2062. Epub 2018 Oct 6. Rejuvenation Res. 2019. PMID: 30091676
-
Naringenin alleviates paraquat-induced dopaminergic neuronal loss in SH-SY5Y cells and a rat model of Parkinson's disease.Neuropharmacology. 2021 Dec 15;201:108831. doi: 10.1016/j.neuropharm.2021.108831. Epub 2021 Oct 13. Neuropharmacology. 2021. PMID: 34655599
-
Orexin-A Protects Human Neuroblastoma SH-SY5Y Cells Against 6-Hydroxydopamine-Induced Neurotoxicity: Involvement of PKC and PI3K Signaling Pathways.Rejuvenation Res. 2017 Apr;20(2):125-133. doi: 10.1089/rej.2016.1836. Epub 2017 Mar 30. Rejuvenation Res. 2017. PMID: 27814668
-
Protective Effect of Neuropeptide Apelin-13 on 6-Hydroxydopamine-Induced Neurotoxicity in SH-SY5Y Dopaminergic Cells: Involvement of Its Antioxidant and Antiapoptotic Properties.Rejuvenation Res. 2018 Apr;21(2):162-167. doi: 10.1089/rej.2017.1951. Epub 2018 Jan 11. Rejuvenation Res. 2018. PMID: 28782414
References
-
- Baltazar M.T., Dinis-Oliveira R.J., Bastos M.L., Tsatsakis A.M., Duarte J.A., Carvalho F. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases–a mechanistic approach. Toxicol. Lett. 2014;230(2):85–103. doi: 10.1016/j.toxlet.2014.01.039. - DOI - PubMed
-
- Biederman J., Boellner S.W., Childress A., Lopez F.A., Krishnan S., Zhang Y. Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: A double-blind, placebo-controlled, crossover analog classroom study. Biol. Psychiatry. 2007;62(9):970–976. doi: 10.1016/j.biopsych.2007.04.015. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

