This is a preprint.
Dynamic PET Reveals Compartmentalized Brain and Lung Tissue Antibiotic Exposures
- PMID: 38562706
- PMCID: PMC10984015
- DOI: 10.21203/rs.3.rs-4096014/v1
Dynamic PET Reveals Compartmentalized Brain and Lung Tissue Antibiotic Exposures
Update in
-
Dynamic PET reveals compartmentalized brain and lung tissue antibiotic exposures of tuberculosis drugs.Nat Commun. 2024 Aug 14;15(1):6657. doi: 10.1038/s41467-024-50989-4. Nat Commun. 2024. PMID: 39143055 Free PMC article.
Abstract
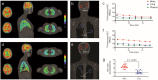
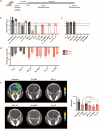
Tuberculosis (TB) remains a leading cause of death, but antibiotic treatments for tuberculous meningitis, the deadliest form of TB, are based on those developed for pulmonary TB and not optimized for brain penetration. Here, we performed first-in-human dynamic 18F-pretomanid positron emission tomography (PET) studies in eight human subjects for three-dimensional, multi-compartmental in situ visualization of antibiotic concentration-time exposures (area under the curve - AUC), demonstrating preferential brain (AUCtissue/plasma 2.25) versus lung (AUCtissue/plasma 0.97) tissue partitioning. Preferential, antibiotic-specific partitioning into brain or lung tissues of antibiotics active against MDR strains were confirmed in experimentally-infected mice and rabbits, using dynamic PET with chemically identical antibiotic radioanalogs, and postmortem mass spectrometry measurements. PET-facilitated pharmacokinetic modeling predicted human dosing necessary to attain therapeutic brain exposures in human subjects. These data were used to design optimized, pretomanid-based regimens which were evaluated at human equipotent dosing in a mouse model of TB meningitis, demonstrating excellent bactericidal activity without an increase in intracerebral inflammation or brain injury. Importantly, several antibiotic regimens demonstrated discordant activities in brain and lung tissues in the same animal, correlating with the compartmentalized tissue exposures of the component antibiotics. These data provide a mechanistic basis for the compartmentalized activities of antibiotic regimens, with important implications for the development of antimicrobial regimens for meningitis and other infections in compartments with unique antibiotic penetration.
Conflict of interest statement
Competing interests Vijay Ivaturi is a co-founder and Vijay Ivaturi and Bhavatharini Arun are employees of Pumas-AI, which commercializes Pumas and Lyv software. All other authors declare that they have no competing interests. Additional Declarations: Yes there is potential Competing Interest. Vijay Ivaturi is a co-founder and Vijay Ivaturi and Bhavatharini Arun are employees of Pumas-AI, which commercializes Pumas and Lyv software. All other authors declare that they have no competing interests.
Figures



References
-
- Ehrlich P Chemotherapeutics: scientific principles, methods, and results. Address in pathology to 17th International Congress of Medicine (London, 1913). Lancet 2, 445–451 (1913).
-
- Wilkinson R.J., et al. Tuberculous meningitis. Nat Rev Neurol 13, 581–598 (2017). - PubMed
-
- Huynh J., et al. Tuberculous meningitis: progress and remaining questions. Lancet Neurol 21, 450–464 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

