This is a preprint.
Dysregulated Ca2+ signaling, fluid secretion, and mitochondrial function in a mouse model of early Sjögren's syndrome
- PMID: 38562738
- PMCID: PMC10983907
- DOI: 10.1101/2024.03.19.585719
Dysregulated Ca2+ signaling, fluid secretion, and mitochondrial function in a mouse model of early Sjögren's syndrome
Update in
-
Dysregulated Ca2+ signaling, fluid secretion, and mitochondrial function in a mouse model of early Sjögren's disease.Elife. 2024 Sep 11;13:RP97069. doi: 10.7554/eLife.97069. Elife. 2024. PMID: 39259200 Free PMC article.
Abstract
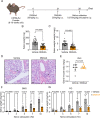
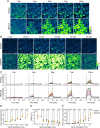
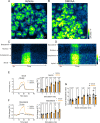
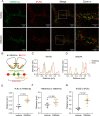
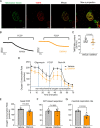
Saliva is essential for oral health. The molecular mechanisms leading to physiological fluid secretion are largely established, but factors that underlie secretory hypofunction, specifically related to the autoimmune disease Sjögren's syndrome (SS) are not fully understood. A major conundrum is the lack of association between the severity of inflammatory immune cell infiltration within the salivary glands and glandular hypofunction. In this study, we investigated in a mouse model system, mechanisms of glandular hypofunction caused by the activation of the stimulator of interferon genes (STING) pathway. Glandular hypofunction and SS-like disease were induced by treatment with 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA), a small molecule agonist of murine STING. Contrary to our expectations, despite a significant reduction in fluid secretion in DMXAA-treated mice, in vivo imaging demonstrated that neural stimulation resulted in greatly enhanced spatially averaged cytosolic Ca2+ levels. Notably, however, the spatiotemporal characteristics of the Ca2+ signals were altered to signals that propagated throughout the entire cytoplasm as opposed to largely apically confined Ca2+ rises observed without treatment. Despite the augmented Ca2+ signals, muscarinic stimulation resulted in reduced activation of TMEM16a, although there were no changes in channel abundance or absolute sensitivity to Ca2+. However, super-resolution microscopy revealed a disruption in the intimate colocalization of Inositol 1,4,5-trisphosphate receptor Ca2+ release channels in relation to TMEM16a. TMEM16a channel activation was also reduced when intracellular Ca2+ buffering was increased. These data are consistent with altered local coupling between the channels contributing to the reduced activation of TMEM16a. Appropriate Ca2+ signaling is also pivotal for mitochondrial morphology and bioenergetics and secretion is an energetically expensive process. Disrupted mitochondrial morphology, a depolarized mitochondrial membrane potential, and reduced oxygen consumption rate were observed in DMXAA-treated animals compared to control animals. We report that early in SS disease, dysregulated Ca2+ signals lead to decreased fluid secretion and disrupted mitochondrial function contributing to salivary gland hypofunction and likely the progression of SS disease.
Keywords: Ca2+ signaling; DMXAA-induced SS mouse model; Salivary gland hypo-function; Sjögren’s syndrome; TMEM16a channel; Xerostomia; mitochondrial dysfunction.
Figures


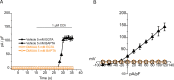


References
-
- Williamson S. P. H. a. R. T. (2001) <A review of saliva-Normal composition, flow, and function.pdf>. J Prosthet Dent 85 - PubMed
-
- Carpenter G. H. (2013) The secretion, components, and properties of saliva. Annu Rev Food Sci Technol 4, 267–276 - PubMed
-
- Pedersen A. M. L., Sorensen C. E., Proctor G. B., Carpenter G. H., and Ekstrom J. (2018) Salivary secretion in health and disease. J Oral Rehabil 45, 730–746 - PubMed
-
- de Paula F., Teshima T. H. N., Hsieh R., Souza M. M., Nico M. M. S., and Lourenco S. V. (2017) Overview of Human Salivary Glands: Highlights of Morphology and Developing Processes. Anat Rec (Hoboken) 300, 1180–1188 - PubMed
-
- Futatsugi A., Nakamura T., Yamada M. K., Ebisui E., Nakamura K., Uchida K., Kitaguchi T., Takahashi-Iwanaga H., Noda T., Aruga J., and Mikoshiba K. (2005) IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 309, 2232–2234 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
