This is a preprint.
Loss of prohibitin 2 in Schwann cells dysregulates key transcription factors controlling developmental myelination
- PMID: 38562812
- PMCID: PMC10983910
- DOI: 10.1101/2024.03.20.585915
Loss of prohibitin 2 in Schwann cells dysregulates key transcription factors controlling developmental myelination
Update in
-
Loss of prohibitin 2 in Schwann cells dysregulates key transcription factors controlling developmental myelination.Glia. 2024 Dec;72(12):2247-2267. doi: 10.1002/glia.24610. Epub 2024 Aug 31. Glia. 2024. PMID: 39215540 Free PMC article.
Abstract
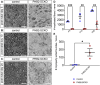
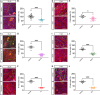
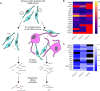
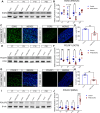
Schwann cells are critical for the proper development and function of the peripheral nervous system, where they form a mutually beneficial relationship with axons. Past studies have highlighted that a pair of proteins called the prohibitins play major roles in Schwann cell biology. Prohibitins are ubiquitously expressed and versatile proteins. We have previously shown that while prohibitins play a crucial role in Schwann cell mitochondria for long-term myelin maintenance and axon health, they may also be present at the Schwann cell-axon interface during development. Here, we expand on this work, showing that drug-mediated modulation of prohibitins in vitro disrupts myelination and confirming that Schwann cell-specific ablation of prohibitin 2 (Phb2) in vivo results in early and severe defects in peripheral nerve development. Using a proteomic approach in vitro, we identify a pool of candidate PHB2 interactors that change their interaction with PHB2 depending on the presence of axonal signals. Furthermore, we show in vivo that loss of Phb2 in mouse Schwann cells causes ineffective proliferation and dysregulation of transcription factors EGR2 (KROX20), POU3F1 (OCT6) and POU3F2 (BRN2) that are necessary for proper Schwann cell maturation. Schwann cell-specific deletion of Jun, a transcription factor associated with negative regulation of myelination, confers partial rescue of the development defect seen in mice lacking Schwann cell Phb2. This work develops our understanding of Schwann cell biology, revealing that Phb2 may directly or indirectly modulate the timely expression of transcription factors necessary for proper peripheral nervous system development, and proposing candidates that may play a role in PHB2-mediated integration of axon signals in the Schwann cell.
Keywords: BAP32; BAP37; BRN2; KROX20; OCT6; REA; c-JUN.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Arthur-Farraj P. J., Latouche M., Wilton D. K., Quintes S., Chabrol E., Banerjee A., Woodhoo A., Jenkins B., Rahman M., Turmaine M., Wicher G. K., Mitter R., Greensmith L., Behrens A., Raivich G., Mirsky R., & Jessen K. R. (2012). c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron, 75(4), 633–647. 10.1016/j.neuron.2012.06.021 - DOI - PMC - PubMed
-
- Arthur-Farraj P. J., Morgan C. C., Adamowicz M., Gomez-Sanchez J. A., Fazal S. V., Beucher A., Razzaghi B., Mirsky R., Jessen K. R., & Aitman T. J. (2017). Changes in the Coding and Non-coding Transcriptome and DNA Methylome that Define the Schwann Cell Repair Phenotype after Nerve Injury. Cell Reports, 20(11), 2719–2734. 10.1016/j.celrep.2017.08.064 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
