This is a preprint.
Genome-wide analyses identify 21 infertility loci and over 400 reproductive hormone loci across the allele frequency spectrum
- PMID: 38562841
- PMCID: PMC10984039
- DOI: 10.1101/2024.03.19.24304530
Genome-wide analyses identify 21 infertility loci and over 400 reproductive hormone loci across the allele frequency spectrum
Update in
-
Genome-wide analyses identify 25 infertility loci and relationships with reproductive traits across the allele frequency spectrum.Nat Genet. 2025 May;57(5):1107-1118. doi: 10.1038/s41588-025-02156-8. Epub 2025 Apr 14. Nat Genet. 2025. PMID: 40229599 Free PMC article.
Abstract
Genome-wide association studies (GWASs) may help inform treatments for infertility, whose causes remain unknown in many cases. Here we present GWAS meta-analyses across six cohorts for male and female infertility in up to 41,200 cases and 687,005 controls. We identified 21 genetic risk loci for infertility (P≤5E-08), of which 12 have not been reported for any reproductive condition. We found positive genetic correlations between endometriosis and all-cause female infertility (r g=0.585, P=8.98E-14), and between polycystic ovary syndrome and anovulatory infertility (r g=0.403, P=2.16E-03). The evolutionary persistence of female infertility-risk alleles in EBAG9 may be explained by recent directional selection. We additionally identified up to 269 genetic loci associated with follicle-stimulating hormone (FSH), luteinising hormone, oestradiol, and testosterone through sex-specific GWAS meta-analyses (N=6,095-246,862). While hormone-associated variants near FSHB and ARL14EP colocalised with signals for anovulatory infertility, we found no r g between female infertility and reproductive hormones (P>0.05). Exome sequencing analyses in the UK Biobank (N=197,340) revealed that women carrying testosterone-lowering rare variants in GPC2 were at higher risk of infertility (OR=2.63, P=1.25E-03). Taken together, our results suggest that while individual genes associated with hormone regulation may be relevant for fertility, there is limited genetic evidence for correlation between reproductive hormones and infertility at the population level. We provide the first comprehensive view of the genetic architecture of infertility across multiple diagnostic criteria in men and women, and characterise its relationship to other health conditions.
Conflict of interest statement
Competing Interests Statement L.B.L.W. is currently employed by Novo Nordisk Research Centre Oxford but, while she conducted the research described in this manuscript, was only affiliated to the University of Oxford. V.S., G.T., H.H., I.J., and K.S. are employees of deCODE genetics, a subsidiary of Amgen. C.M.L. reports grants from Bayer AG and Novo Nordisk and has a partner who works at Vertex. The other authors declare no conflicts of interest.
Figures
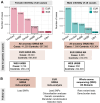
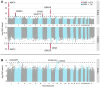




References
-
- Infertility prevalence estimates, 1990–2021. https://www.who.int/publications/i/item/978920068315 (2023).
-
- Brandt J. S., Cruz Ithier M. A., Rosen T. & Ashkinadze E. Advanced paternal age, infertility, and reproductive risks: A review of the literature. Prenat. Diagn. 39, 81–87 (2019). - PubMed
-
- Crawford N. M. & Steiner A. Z. Age-related infertility. Obstet. Gynecol. Clin. North Am. 42, 15–25 (2015). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
