This is a preprint.
Host cell-specific metabolism of linoleic acid controls Toxoplasma gondii growth in cell culture
- PMID: 38562845
- PMCID: PMC10983968
- DOI: 10.1101/2024.03.22.586332
Host cell-specific metabolism of linoleic acid controls Toxoplasma gondii growth in cell culture
Update in
-
Host cell-specific metabolism of linoleic acid controls Toxoplasma gondii growth in cell culture.Infect Immun. 2024 Oct 15;92(10):e0029924. doi: 10.1128/iai.00299-24. Epub 2024 Aug 28. Infect Immun. 2024. PMID: 39194219 Free PMC article.
Abstract
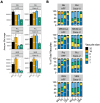
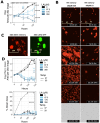
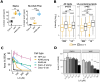
The obligate intracellular parasite Toxoplasma gondii can infect and replicate in any warm-blooded cell tested to date, but much of our knowledge about T. gondii cell biology comes from just one host cell type: human foreskin fibroblasts (HFFs). To expand our knowledge of host-parasite lipid interactions, we studied T. gondii in intestinal epithelial cells, the first site of host-parasite contact following oral infection and the exclusive site of parasite sexual development in feline hosts. We found that highly metabolic Caco-2 cells are permissive to T. gondii growth even when treated with high levels of linoleic acid (LA), a polyunsaturated fatty acid (PUFA) that kills parasites in HFFs. Caco-2 cells appear to sequester LA away from the parasite, preventing membrane disruptions and lipotoxicity that characterize LA-induced parasite death in HFFs. Our work is an important step toward understanding host-parasite interactions in feline intestinal epithelial cells, an understudied but important cell type in the T. gondii life cycle.
Keywords: Toxoplasma gondii; enterocyte; linoleic acid; lipid droplet; lipotoxicity; polyunsaturated fatty acid.
Conflict of interest statement
Declaration of Interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Tomasina Ramiro, and Francia María E.. “The Structural and Molecular Underpinnings of Gametogenesis in Toxoplasma Gondii.” Frontiers in Cellular and Infection Microbiology, vol. 10, 2020. Frontiers, https://www.frontiersin.org/articles/. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
