This is a preprint.
Functional Redundancy in Candida auris Cell Surface Adhesins Crucial for Cell-Cell Interaction and Aggregation
- PMID: 38562859
- PMCID: PMC10984083
- DOI: 10.21203/rs.3.rs-4077218/v1
Functional Redundancy in Candida auris Cell Surface Adhesins Crucial for Cell-Cell Interaction and Aggregation
Update in
-
Functional redundancy in Candida auris cell surface adhesins crucial for cell-cell interaction and aggregation.Nat Commun. 2024 Oct 25;15(1):9212. doi: 10.1038/s41467-024-53588-5. Nat Commun. 2024. PMID: 39455573 Free PMC article.
Abstract
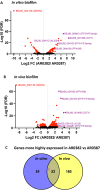
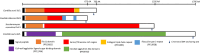
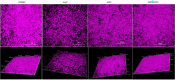
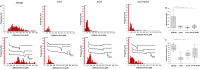
Candida auris is an emerging nosocomial fungal pathogen associated with life-threatening invasive disease due to its persistent colonization, high level of transmissibility and multi-drug resistance. Aggregative and non-aggregative growth phenotypes for C. auris strains with different biofilm forming abilities, drug susceptibilities and virulence characteristics have been described. Using comprehensive transcriptional analysis we identified key cell surface adhesins that were highly upregulated in the aggregative phenotype during in vitro and in vivo grown biofilms using a mouse model of catheter infection. Phenotypic and functional evaluations of generated null mutants demonstrated crucial roles for the adhesins Als5 and Scf1 in mediating cell-cell adherence, coaggregation and biofilm formation. While individual mutants were largely non-aggregative, in combination cells were able to co-adhere and aggregate, as directly demonstrated by measuring cell adhesion forces using single-cell atomic force spectroscopy. This co-adherence indicates their role as complementary adhesins, which despite their limited similarity, may function redundantly to promote cell-cell interaction and biofilm formation. Functional diversity of cell wall proteins may be a form of regulation that provides the aggregative phenotype of C. auris with flexibility and rapid adaptation to the environment, potentially impacting persistence and virulence.
Conflict of interest statement
Additional Declarations: There is NO Competing Interest.
Figures



Similar articles
-
Functional Redundancy in Candida auris Cell Surface Adhesins Crucial for Cell-Cell Interaction and Aggregation.bioRxiv [Preprint]. 2024 Mar 21:2024.03.21.586120. doi: 10.1101/2024.03.21.586120. bioRxiv. 2024. Update in: Nat Commun. 2024 Oct 25;15(1):9212. doi: 10.1038/s41467-024-53588-5. PMID: 38562758 Free PMC article. Updated. Preprint.
-
Functional redundancy in Candida auris cell surface adhesins crucial for cell-cell interaction and aggregation.Nat Commun. 2024 Oct 25;15(1):9212. doi: 10.1038/s41467-024-53588-5. Nat Commun. 2024. PMID: 39455573 Free PMC article.
-
Biofilm formation by Candida auris isolated from colonising sites and candidemia cases.Mycoses. 2019 Aug;62(8):706-709. doi: 10.1111/myc.12947. Epub 2019 Jun 9. Mycoses. 2019. PMID: 31132181
-
Intra-clade Heterogeneity in Candida auris: Risk of Management.Curr Microbiol. 2023 Jul 24;80(9):295. doi: 10.1007/s00284-023-03416-8. Curr Microbiol. 2023. PMID: 37486431 Review.
-
Mechanisms of pathogenicity for the emerging fungus Candida auris.PLoS Pathog. 2023 Dec 21;19(12):e1011843. doi: 10.1371/journal.ppat.1011843. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38127686 Free PMC article. Review.
References
-
- de Jong A.W. & Hagen F. Attack, defend and persist: How the fungal pathogen Candida auris was able to emerge globally in healthcare environments. Mycopathologia. 148, 353–365. (2019). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

