Improved Thermal Stability of a Novel Acidophilic Phytase
- PMID: 38563103
- PMCID: PMC11180912
- DOI: 10.4014/jmb.2311.11044
Improved Thermal Stability of a Novel Acidophilic Phytase
Abstract
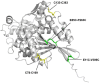
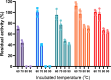
Phytase increases the availability of phosphate and trace elements by hydrolyzing the phosphomonoester bond in phytate present in animal feed. It is also an important enzyme from an environmental perspective because it not only promotes the growth of livestocks but also prevents phosphorus contamination released into the environment. Here we present a novel phytase derived from Turicimonas muris, TmPhy, which has distinctive structure and properties compared to other previously known phytases. TmPhy gene expressed in the Pichia system was confirmed to be 41 kDa in size and was used in purified form to evaluate optimal conditions for maximum activity. TmPhy has a dual optimum pH at pH3 and pH6.8 and exhibited the highest activity at 70°C. However, the heat tolerance of the wildtype was not satisfactory for feed application. Therefore, random mutation, disulfide bond introduction, and N-terminal mutation were performed to improve the thermostability of the TmPhy. Random mutation resulted in TmPhyM with about 45% improvement in stability at 60°C. Through further improvements, a total of three mutants were screened and their heat tolerance was evaluated. As a result, we obtained TmPhyMD1 with 46.5% residual activity, TmPhyMD2 with 74.1%, and TmPhyMD3 with 66.8% at 80°C heat treatment without significant loss of or with increased activity.
Keywords: Acidophilic; dual optimum pH; phytase; phytic acid; thermostable.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures

Similar articles
-
A Thermostable phytase from Neosartorya spinosa BCC 41923 and its expression in Pichia pastoris.J Microbiol. 2011 Apr;49(2):257-64. doi: 10.1007/s12275-011-0369-x. Epub 2011 May 3. J Microbiol. 2011. PMID: 21538247
-
Molecular advancements in the development of thermostable phytases.Appl Microbiol Biotechnol. 2017 Apr;101(7):2677-2689. doi: 10.1007/s00253-017-8195-7. Epub 2017 Feb 23. Appl Microbiol Biotechnol. 2017. PMID: 28233043 Review.
-
Expression and characterization of Aspergillus thermostable phytases in Pichia pastoris.FEMS Microbiol Lett. 2009 Jan;290(1):18-24. doi: 10.1111/j.1574-6968.2008.01399.x. Epub 2008 Nov 12. FEMS Microbiol Lett. 2009. PMID: 19025560
-
Enhancement of thermostability and kinetic efficiency of Aspergillus niger PhyA phytase by site-directed mutagenesis.Appl Biochem Biotechnol. 2015 Mar;175(5):2528-41. doi: 10.1007/s12010-014-1440-y. Epub 2014 Dec 20. Appl Biochem Biotechnol. 2015. PMID: 25527139
-
Advances in phytase research.Adv Appl Microbiol. 2000;47:157-99. doi: 10.1016/s0065-2164(00)47004-8. Adv Appl Microbiol. 2000. PMID: 12876797 Review.
Cited by
-
Modification and applications of glucose oxidase: optimization strategies and high-throughput screening technologies.World J Microbiol Biotechnol. 2025 Jul 12;41(7):266. doi: 10.1007/s11274-025-04475-8. World J Microbiol Biotechnol. 2025. PMID: 40650823 Review.
References
-
- Hídvégi M, Lásztity R. Phytic acid content of cereals and legumes and interaction with proteins. Periodica Polytechnica Ser. Chem. Eng. 2002;46:56–94.
-
- Selle PH, Ravindran V. Phytate-degrading enzymes in pig nutrition. Livest. Sci. 2008;113:99–122. doi: 10.1016/j.livsci.2007.05.014. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

