Age-Dependent RGS5 Loss in Pericytes Induces Cardiac Dysfunction and Fibrosis
- PMID: 38563133
- PMCID: PMC11081481
- DOI: 10.1161/CIRCRESAHA.123.324183
Age-Dependent RGS5 Loss in Pericytes Induces Cardiac Dysfunction and Fibrosis
Abstract
Background: Pericytes are capillary-associated mural cells involved in the maintenance and stability of the vascular network. Although aging is one of the main risk factors for cardiovascular disease, the consequences of aging on cardiac pericytes are unknown.
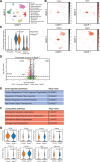
Methods: In this study, we have combined single-nucleus RNA sequencing and histological analysis to determine the effects of aging on cardiac pericytes. Furthermore, we have conducted in vivo and in vitro analysis of RGS5 (regulator of G-protein signaling 5) loss of function and finally have performed pericytes-fibroblasts coculture studies to understand the effect of RGS5 deletion in pericytes on the neighboring fibroblasts.
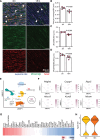
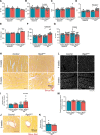
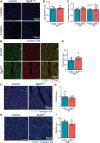
Results: Aging reduced the pericyte area and capillary coverage in the murine heart. Single-nucleus RNA sequencing analysis further revealed that the expression of Rgs5 was reduced in cardiac pericytes from aged mice. In vivo and in vitro studies showed that the deletion of RGS5 impaired cardiac function, induced fibrosis, and morphological changes in pericytes characterized by a profibrotic gene expression signature and the expression of different ECM (extracellular matrix) components and growth factors, for example, TGFB2 and PDGFB. Indeed, culturing fibroblasts with the supernatant of RGS5-deficient pericytes induced their activation as evidenced by the increased expression of αSMA (alpha smooth muscle actin) in a TGFβ (transforming growth factor beta)2-dependent mechanism.
Conclusions: Our results have identified RGS5 as a crucial regulator of pericyte function during cardiac aging. The deletion of RGS5 causes cardiac dysfunction and induces myocardial fibrosis, one of the hallmarks of cardiac aging.
Keywords: cardiovascular diseases; fibroblasts; fibrosis; heart failure; pericytes.
Conflict of interest statement
Figures



Comment in
-
Keeps Cardiac Pericytes in Good Shape: Regulator of G-Protein Signaling-5.Circ Res. 2024 May 10;134(10):1256-1258. doi: 10.1161/CIRCRESAHA.124.324476. Epub 2024 May 9. Circ Res. 2024. PMID: 38723034 No abstract available.
References
-
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7 - PubMed
-
- Holm A, Heumann T, Augustin HG. Microvascular mural cell organotypic heterogeneity and functional plasticity. Trends Cell Biol. 2018;28:302–316. doi: 10.1016/j.tcb.2017.12.002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

