Regulation of ubiquitination and antiviral activity of Cactin by deubiquitinase Usp14 in Drosophila
- PMID: 38563731
- PMCID: PMC11092352
- DOI: 10.1128/jvi.00177-24
Regulation of ubiquitination and antiviral activity of Cactin by deubiquitinase Usp14 in Drosophila
Abstract
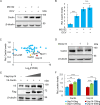
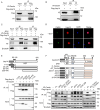
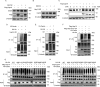
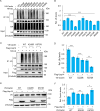
Cactin, a highly conserved protein, plays a crucial role in various physiological processes in eukaryotes, including innate immunity. Recently, the function of Cactin in the innate immunity of Drosophila has been explored, revealing that Cactin regulates a non-canonical signaling pathway associated with the Toll and Imd pathways via the Cactin-Deaf1 axis. In addition, Cactin exhibits specific antiviral activity against the Drosophila C virus (DCV) in Drosophila, with an unknown mechanism. During DCV infection, it has been confirmed that the protein level and antiviral activity of Cactin are regulated by ubiquitination. However, the precise ubiquitination and deubiquitination mechanisms of Cactin in Drosophila remain unexplored. In this study, we identified ubiquitin-specific protease 14 (Usp14) as a major deubiquitinase for Cactin through comprehensive deubiquitinase screening. Our results demonstrate that Usp14 interacts with the C_Cactus domain of Cactin via its USP domain. Usp14 efficiently removes K48- and K63-linked polyubiquitin chains from Cactin, thereby preventing its degradation through the ubiquitin-proteasome pathway. Usp14 significantly inhibits DCV replication in Drosophila cells by stabilizing Cactin. Moreover, Usp14-deficient fruit flies exhibit increased susceptibility to DCV infection compared to wild-type flies. Collectively, our findings reveal the regulation of ubiquitination and antiviral activity of Cactin by the deubiquitinase Usp14, providing valuable insights into the modulation of Cactin-mediated antiviral activity in Drosophila.IMPORTANCEViral infections pose a severe threat to human health, marked by high pathogenicity and mortality rates. Innate antiviral pathways, such as Toll, Imd, and JAK-STAT, are generally conserved across insects and mammals. Recently, the multi-functionality of Cactin in innate immunity has been identified in Drosophila. In addition to regulating a non-canonical signaling pathway through the Cactin-Deaf1 axis, Cactin exhibits specialized antiviral activity against the Drosophila C virus (DCV) with an unknown mechanism. A previous study emphasized the significance of the Cactin level, regulated by the ubiquitin-proteasome pathway, in modulating antiviral signaling. However, the regulatory mechanisms governing Cactin remain unexplored. In this study, we demonstrate that Usp14 stabilizes Cactin by preventing its ubiquitination and subsequent degradation. Furthermore, Usp14 plays a crucial role in regulating the antiviral function mediated by Cactin. Therefore, our findings elucidate the regulatory mechanism of Cactin in Drosophila, offering a potential target for the prevention and treatment of viral infections.
Keywords: Cactin; Drosophila; antiviral immunity; deubiquitinase; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Drosophila melanogaster Toll-9 elicits antiviral immunity against Drosophila C virus.J Virol. 2025 Jun 17;99(6):e0221424. doi: 10.1128/jvi.02214-24. Epub 2025 May 14. J Virol. 2025. PMID: 40366172 Free PMC article.
-
Regulation of phase separation and antiviral activity of Cactin by glycolytic enzyme PGK via phosphorylation in Drosophila.mBio. 2024 Apr 10;15(4):e0137823. doi: 10.1128/mbio.01378-23. Epub 2024 Mar 6. mBio. 2024. PMID: 38446061 Free PMC article.
-
lncRNA Sensing of a Viral Suppressor of RNAi Activates Non-canonical Innate Immune Signaling in Drosophila.Cell Host Microbe. 2020 Jan 8;27(1):115-128.e8. doi: 10.1016/j.chom.2019.12.006. Cell Host Microbe. 2020. PMID: 31917956
-
Ubiquitin signalling in Drosophila innate immune responses.FEBS J. 2024 Oct;291(20):4397-4413. doi: 10.1111/febs.17028. Epub 2023 Dec 18. FEBS J. 2024. PMID: 38069549 Review.
-
Ubiquitin-specific protease 14 is a new therapeutic target for the treatment of diseases.J Cell Physiol. 2021 May;236(5):3396-3405. doi: 10.1002/jcp.30124. Epub 2020 Nov 1. J Cell Physiol. 2021. PMID: 33135160 Review.
Cited by
-
OTULIN Interactome Reveals Immune Response and Autophagy Associated with Tauopathy in a Mouse Model.bioRxiv [Preprint]. 2025 Feb 8:2025.02.07.636114. doi: 10.1101/2025.02.07.636114. bioRxiv. 2025. PMID: 39974971 Free PMC article. Preprint.
References
-
- Wallace MA, Coffman KA, Gilbert C, Ravindran S, Albery GF, Abbott J, Argyridou E, Bellosta P, Betancourt AJ, Colinet H, et al. . 2021. The discovery, distribution, and diversity of DNA viruses associated with Drosophila melanogaster in Europe. Virus Evol 7:veab031. doi:10.1093/ve/veab031 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

