The Ca2+-dependent phosphatase calcineurin dephosphorylates TBK1 to suppress antiviral innate immunity
- PMID: 38563732
- PMCID: PMC11092360
- DOI: 10.1128/jvi.00016-24
The Ca2+-dependent phosphatase calcineurin dephosphorylates TBK1 to suppress antiviral innate immunity
Abstract
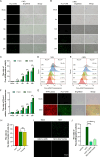
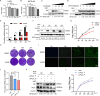
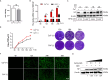
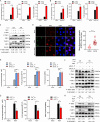
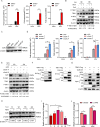
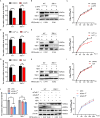
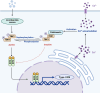
Tumor necrosis factor receptor-associated factor family member-associated NF-κB activator-binding kinase 1 (TBK1) plays a key role in the induction of the type 1 interferon (IFN-I) response, which is an important component of innate antiviral defense. Viruses target calcium (Ca2+) signaling networks, which participate in the regulation of the viral life cycle, as well as mediate the host antiviral response. Although many studies have focused on the role of Ca2+ signaling in the regulation of IFN-I, the relationship between Ca2+ and TBK1 in different infection models requires further elucidation. Here, we examined the effects of the Newcastle disease virus (NDV)-induced increase in intracellular Ca2+ levels on the suppression of host antiviral responses. We demonstrated that intracellular Ca2+ increased significantly during NDV infection, leading to impaired IFN-I production and antiviral immunity through the activation of calcineurin (CaN). Depletion of Ca²+ was found to lead to a significant increase in virus-induced IFN-I production resulting in the inhibition of viral replication. Mechanistically, the accumulation of Ca2+ in response to viral infection increases the phosphatase activity of CaN, which in turn dephosphorylates and inactivates TBK1 in a Ca2+-dependent manner. Furthermore, the inhibition of CaN on viral replication was counteracted in TBK1 knockout cells. Together, our data demonstrate that NDV hijacks Ca2+ signaling networks to negatively regulate innate immunity via the CaN-TBK1 signaling axis. Thus, our findings not only identify the mechanism by which viruses exploit Ca2+ signaling to evade the host antiviral response but also, more importantly, highlight the potential role of Ca2+ homeostasis in the viral innate immune response.IMPORTANCEViral infections disrupt intracellular Ca2+ homeostasis, which affects the regulation of various host processes to create conditions that are conducive for their own proliferation, including the host immune response. The mechanism by which viruses trigger TBK1 activation and IFN-I induction through viral pathogen-associated molecular patterns has been well defined. However, the effects of virus-mediated Ca2+ imbalance on the IFN-I pathway requires further elucidation, especially with respect to TBK1 activation. Herein, we report that NDV infection causes an increase in intracellular free Ca2+ that leads to activation of the serine/threonine phosphatase CaN, which subsequently dephosphorylates TBK1 and negatively regulates IFN-I production. Furthermore, depletion of Ca2+ or inhibition of CaN activity exerts antiviral effects by promoting the production of IFN-I and inhibiting viral replication. Thus, our results reveal the potential role of Ca2+ in the innate immune response to viruses and provide a theoretical reference for the treatment of viral infectious diseases.
Keywords: Ca2+; TBK1; antiviral immunity; calcineurin; virus infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Phosphatase Cdc25A Negatively Regulates the Antiviral Immune Response by Inhibiting TBK1 Activity.J Virol. 2018 Sep 12;92(19):e01118-18. doi: 10.1128/JVI.01118-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021902 Free PMC article.
-
Kisspeptin/GPR54 signaling restricts antiviral innate immune response through regulating calcineurin phosphatase activity.Sci Adv. 2018 Aug 8;4(8):eaas9784. doi: 10.1126/sciadv.aas9784. eCollection 2018 Aug. Sci Adv. 2018. PMID: 30101190 Free PMC article.
-
The Kinase MAP4K1 Inhibits Cytosolic RNA-Induced Antiviral Signaling by Promoting Proteasomal Degradation of TBK1/IKKε.Microbiol Spectr. 2021 Dec 22;9(3):e0145821. doi: 10.1128/Spectrum.01458-21. Epub 2021 Dec 15. Microbiol Spectr. 2021. PMID: 34908452 Free PMC article.
-
TANK-binding kinase 1 as a novel therapeutic target for viral diseases.Expert Opin Ther Targets. 2019 May;23(5):437-446. doi: 10.1080/14728222.2019.1601702. Epub 2019 Apr 10. Expert Opin Ther Targets. 2019. PMID: 30932713 Review.
-
Negative regulation of TBK1-mediated antiviral immunity.FEBS Lett. 2013 Mar 18;587(6):542-8. doi: 10.1016/j.febslet.2013.01.052. Epub 2013 Feb 8. FEBS Lett. 2013. PMID: 23395611 Free PMC article. Review.
References
-
- Friedman CS, O’Donnell MA, Legarda-Addison D, Ng A, Cárdenas WB, Yount JS, Moran TM, Basler CF, Komuro A, Horvath CM, Xavier R, Ting AT. 2008. The tumour suppressor CYLD is a negative regulator of RIG-I-mediated antiviral response. EMBO Rep. 9:930–936. doi:10.1038/embor.2008.136 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 32122085/MOST | National Natural Science Foundation of China (NSFC)
- 32030108/MOST | National Natural Science Foundation of China (NSFC)
- 32220103012/International Cooperation Project of National Natural Science Foundation of China
- 2022YFD1801500/MOST | National Key Research and Development Program of China (NKPs)
LinkOut - more resources
Full Text Sources
Miscellaneous

