Tetrahydrofolate levels influence 2-aminoacrylate stress in Salmonella enterica
- PMID: 38563759
- PMCID: PMC11025330
- DOI: 10.1128/jb.00042-24
Tetrahydrofolate levels influence 2-aminoacrylate stress in Salmonella enterica
Abstract
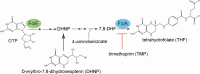
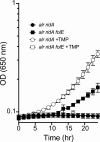
In Salmonella enterica, the absence of the RidA deaminase results in the accumulation of the reactive enamine 2-aminoacrylate (2AA). The resulting 2AA stress impacts metabolism and prevents growth in some conditions by inactivating a specific target pyridoxal 5'-phosphate (PLP)-dependent enzyme(s). The detrimental effects of 2AA stress can be overcome by changing the sensitivity of a critical target enzyme or modifying flux in one or more nodes in the metabolic network. The catabolic L-alanine racemase DadX is a target of 2AA, which explains the inability of an alr ridA strain to use L-alanine as the sole nitrogen source. Spontaneous mutations that suppressed the growth defect of the alr ridA strain were identified as lesions in folE, which encodes GTP cyclohydrolase and catalyzes the first step of tetrahydrofolate (THF) synthesis. The data here show that THF limitation resulting from a folE lesion, or inhibition of dihydrofolate reductase (FolA) by trimethoprim, decreases the 2AA generated from endogenous serine. The data are consistent with an increased level of threonine, resulting from low folate levels, decreasing 2AA stress.IMPORTANCERidA is an enamine deaminase that has been characterized as preventing the 2-aminoacrylate (2AA) stress. In the absence of RidA, 2AA accumulates and damages various cellular enzymes. Much of the work describing the 2AA stress system has depended on the exogenous addition of serine to increase the production of the enamine stressor. The work herein focuses on understanding the effect of 2AA stress generated from endogenous serine pools. As such, this work describes the consequences of a subtle level of stress that nonetheless compromises growth in at least two conditions. Describing mechanisms that alter the physiological consequences of 2AA stress increases our understanding of endogenous metabolic stress and how the robustness of the metabolic network allows perturbations to be modulated.
Keywords: 2-aminoacrylate stress; RidA; alanine racemase; folate biosynthesis; metabolic integration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Expression of Pyridoxal 5'-Phosphate-Independent Racemases Can Reduce 2-Aminoacrylate Stress in Salmonella enterica.J Bacteriol. 2018 Apr 9;200(9):e00751-17. doi: 10.1128/JB.00751-17. Print 2018 May 1. J Bacteriol. 2018. PMID: 29440254 Free PMC article.
-
The Cysteine Desulfurase IscS Is a Significant Target of 2-Aminoacrylate Damage in Pseudomonas aeruginosa.mBio. 2022 Jun 28;13(3):e0107122. doi: 10.1128/mbio.01071-22. Epub 2022 Jun 2. mBio. 2022. PMID: 35652590 Free PMC article.
-
2-Aminoacrylate stress damages diverse PLP-dependent enzymes in vivo.J Biol Chem. 2022 Jun;298(6):101970. doi: 10.1016/j.jbc.2022.101970. Epub 2022 Apr 20. J Biol Chem. 2022. PMID: 35460692 Free PMC article.
-
From microbiology to cancer biology: the Rid protein family prevents cellular damage caused by endogenously generated reactive nitrogen species.Mol Microbiol. 2015 Apr;96(2):211-9. doi: 10.1111/mmi.12945. Epub 2015 Feb 26. Mol Microbiol. 2015. PMID: 25620221 Free PMC article. Review.
-
RidA Proteins Protect against Metabolic Damage by Reactive Intermediates.Microbiol Mol Biol Rev. 2020 Jul 15;84(3):e00024-20. doi: 10.1128/MMBR.00024-20. Print 2020 Aug 19. Microbiol Mol Biol Rev. 2020. PMID: 32669283 Free PMC article. Review.
Cited by
-
Perturbation of the MetJ regulon impacts the consequences of 2-aminoacrylate stress in Salmonella enterica.Microbiology (Reading). 2025 Jun;171(6):001572. doi: 10.1099/mic.0.001572. Microbiology (Reading). 2025. PMID: 40552973 Free PMC article.
-
Exploring the bioactive compounds of Carica papaya leaves: phytol's role in combatting antibiotic-resistant bacteria.Front Cell Infect Microbiol. 2025 Jul 7;15:1564787. doi: 10.3389/fcimb.2025.1564787. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40692687 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

