A measles-vectored vaccine candidate expressing prefusion-stabilized SARS-CoV-2 spike protein brought to phase I/II clinical trials: protection of African green monkeys from COVID-19 disease
- PMID: 38563762
- PMCID: PMC11092351
- DOI: 10.1128/jvi.01762-23
A measles-vectored vaccine candidate expressing prefusion-stabilized SARS-CoV-2 spike protein brought to phase I/II clinical trials: protection of African green monkeys from COVID-19 disease
Abstract
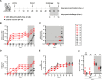
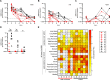
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged at the end of 2019 and is responsible for the largest human pandemic in 100 years. Thirty-four vaccines are currently approved for use worldwide, and approximately 67% of the world population has received a complete primary series of one, yet countries are dealing with new waves of infections, variant viruses continue to emerge, and breakthrough infections are frequent secondary to waning immunity. Here, we evaluate a measles virus (MV)-vectored vaccine expressing a stabilized prefusion SARS-CoV-2 spike (S) protein (MV-ATU3-S2PΔF2A; V591) with demonstrated immunogenicity in mouse models (see companion article [J. Brunet, Z. Choucha, M. Gransagne, H. Tabbal, M.-W. Ku et al., J Virol 98:e01693-23, 2024, https://doi.org/10.1128/jvi.01693-23]) in an established African green monkey model of disease. Animals were vaccinated with V591 or the control vaccine (an equivalent MV-vectored vaccine with an irrelevant antigen) intramuscularly using a prime/boost schedule, followed by challenge with an early pandemic isolate of SARS-CoV-2 at 56 days post-vaccination. Pre-challenge, only V591-vaccinated animals developed S-specific antibodies that had virus-neutralizing activity as well as S-specific T cells. Following the challenge, V591-vaccinated animals had lower infectious virus and viral (v) RNA loads in mucosal secretions and stopped shedding virus in these secretions earlier. vRNA loads were lower in these animals in respiratory and gastrointestinal tract tissues at necropsy. This correlated with a lower disease burden in the lungs as quantified by PET/CT at early and late time points post-challenge and by pathological analysis at necropsy.IMPORTANCESevere acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the largest human pandemic in 100 years. Even though vaccines are currently available, countries are dealing with new waves of infections, variant viruses continue to emerge, breakthrough infections are frequent, and vaccine hesitancy persists. This study uses a safe and effective measles vaccine as a platform for vaccination against SARS-CoV-2. The candidate vaccine was used to vaccinate African green monkeys (AGMs). All vaccinated AGMs developed robust antigen-specific immune responses. After challenge, these AGMs produced less virus in mucosal secretions, for a shorter period, and had a reduced disease burden in the lungs compared to control animals. At necropsy, lower levels of viral RNA were detected in tissue samples from vaccinated animals, and the lungs of these animals lacked the histologic hallmarks of SARS-CoV-2 disease observed exclusively in the control AGMs.
Keywords: SARS-CoV-2; measles-vectored vaccine; non-human primates; vectored vaccines.
Conflict of interest statement
C.G., A.M., and N.E. are inventors on a patent application describing measles-vectored vaccine candidates against SARS-CoV-2. All other authors declare no financial or non-financial competing interests.
Figures




References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team . 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi:10.1056/NEJMoa2001017 - DOI - PMC - PubMed
-
- World Health Organization . 2020. WHO director-general’s opening remarks at the media briefing on COVID-19. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...
-
- World Health Organization . 2023. WHO coronavirus (COVID-19) dashboard. Available from: https://covid19.who.int/
-
- World Health Organization . 2021. Classification of Omicron (B11529): SARS-CoV-2 variant of concern. Available from: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

