A measles-vectored vaccine candidate expressing prefusion-stabilized SARS-CoV-2 spike protein brought to phase I/II clinical trials: candidate selection in a preclinical murine model
- PMID: 38563763
- PMCID: PMC11210269
- DOI: 10.1128/jvi.01693-23
A measles-vectored vaccine candidate expressing prefusion-stabilized SARS-CoV-2 spike protein brought to phase I/II clinical trials: candidate selection in a preclinical murine model
Abstract
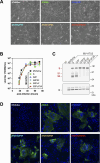
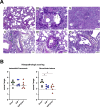
In the early COVID-19 pandemic with urgent need for countermeasures, we aimed at developing a replicating viral vaccine using the highly efficacious measles vaccine as vector, a promising technology with prior clinical proof of concept. Building on our successful pre-clinical development of a measles virus (MV)-based vaccine candidate against the related SARS-CoV, we evaluated several recombinant MV expressing codon-optimized SARS-CoV-2 spike glycoprotein. Candidate V591 expressing a prefusion-stabilized spike through introduction of two proline residues in HR1 hinge loop, together with deleted S1/S2 furin cleavage site and additional inactivation of the endoplasmic reticulum retrieval signal, was the most potent in eliciting neutralizing antibodies in mice. After single immunization, V591 induced similar neutralization titers as observed in sera of convalescent patients. The cellular immune response was confirmed to be Th1 skewed. V591 conferred long-lasting protection against SARS-CoV-2 challenge in a murine model with marked decrease in viral RNA load, absence of detectable infectious virus loads, and reduced lesions in the lungs. V591 was furthermore efficacious in an established non-human primate model of disease (see companion article [S. Nambulli, N. Escriou, L. J. Rennick, M. J. Demers, N. L. Tilston-Lunel et al., J Virol 98:e01762-23, 2024, https://doi.org/10.1128/jvi.01762-23]). Thus, V591 was taken forward into phase I/II clinical trials in August 2020. Unexpected low immunogenicity in humans (O. Launay, C. Artaud, M. Lachâtre, M. Ait-Ahmed, J. Klein et al., eBioMedicine 75:103810, 2022, https://doi.org/10.1016/j.ebiom.2021.103810) revealed that the underlying mechanisms for resistance or sensitivity to pre-existing anti-measles immunity are not yet understood. Different hypotheses are discussed here, which will be important to investigate for further development of the measles-vectored vaccine platform.IMPORTANCESARS-CoV-2 emerged at the end of 2019 and rapidly spread worldwide causing the COVID-19 pandemic that urgently called for vaccines. We developed a vaccine candidate using the highly efficacious measles vaccine as vector, a technology which has proved highly promising in clinical trials for other pathogens. We report here and in the companion article by Nambulli et al. (J Virol 98:e01762-23, 2024, https://doi.org/10.1128/jvi.01762-23) the design, selection, and preclinical efficacy of the V591 vaccine candidate that was moved into clinical development in August 2020, 7 months after the identification of SARS-CoV-2 in Wuhan. These unique in-human trials of a measles vector-based COVID-19 vaccine revealed insufficient immunogenicity, which may be the consequence of previous exposure to the pediatric measles vaccine. The three studies together in mice, primates, and humans provide a unique insight into the measles-vectored vaccine platform, raising potential limitations of surrogate preclinical models and calling for further refinement of the platform.
Keywords: COVID-19 vaccine; measles vector; prefusion-stabilized spike; vectored vaccine.
Conflict of interest statement
J. Brunet, Z.C., M.G., H.S.-M., M.M., P.C., L. Majlessi, C.G., A.M., and N.E. are inventors of a patent application describing measles-vectored vaccine candidates against SARS-CoV-2. All other authors declare no financial or non-financial competing interests.
Figures






References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team . 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi:10.1056/NEJMoa2001017 - DOI - PMC - PubMed
-
- World Health Organization . 2023. WHO COVID-19 dashboard. Available from: https://covid19.who.int. Retrieved 27 Jul 2023.
-
- World Health Organization . 2023. WHO director-general's opening remarks at the media briefing. Available from: https://www.who.int/news-room/speeches/item/who-director-general-s-openi.... Retrieved 27 Jul 2023.
-
- Basta NE, Moodie EMM, on behalf of the VIPER (Vaccines IdP, and Epidemiology Research) Group COVID-19 Vaccine Development and Approvals Tracker Team . 2022. COVID-19 vaccine development and approvals tracker. Available from: https://covid19.trackvaccines.org/vaccines/approved/#vaccine-list. Retrieved 27 Jul 2023.
MeSH terms
Substances
Grants and funding
- Outbreak Response to Novel Coronavirus (COVID-19): Development of a vaccine against novel coronavirus SARS-CoV-2 using the clinical Phase III measles vector technology/Coalition for Epidemic Preparedness Innovations (CEPI)
- 101003589 (post-doctoral fellowship)/EC | Horizon 2020 Framework Programme (H2020)
- 2 post-doctoral fellowships/Agence Nationale de la Recherche (ANR)
- UC7 AI180311/AI/NIAID NIH HHS/United States
- doctoral fellowship/Université Sorbonne Paris Cité (USPC)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

