Testing multiplexed anti-ASFV CRISPR-Cas9 in reducing African swine fever virus
- PMID: 38563791
- PMCID: PMC11218517
- DOI: 10.1128/spectrum.02164-23
Testing multiplexed anti-ASFV CRISPR-Cas9 in reducing African swine fever virus
Abstract
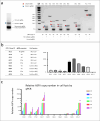
African swine fever (ASF) is a highly fatal viral disease that poses a significant threat to domestic pigs and wild boars globally. In our study, we aimed to explore the potential of a multiplexed CRISPR-Cas system in suppressing ASFV replication and infection. By engineering CRISPR-Cas systems to target nine specific loci within the ASFV genome, we observed a substantial reduction in viral replication in vitro. This reduction was achieved through the concerted action of both Type II and Type III RNA polymerase-guided gRNA expression. To further evaluate its anti-viral function in vivo, we developed a pig strain expressing the multiplexable CRISPR-Cas-gRNA via germline genome editing. These transgenic pigs exhibited normal health with continuous expression of the CRISPR-Cas-gRNA system, and a subset displayed latent viral replication and delayed infection. However, the CRISPR-Cas9-engineered pigs did not exhibit a survival advantage upon exposure to ASFV. To our knowledge, this study represents the first instance of a living organism engineered via germline editing to assess resistance to ASFV infection using a CRISPR-Cas system. Our findings contribute valuable insights to guide the future design of enhanced viral immunity strategies.
Importance: ASFV is currently a devastating disease with no effective vaccine or treatment available. Our study introduces a multiplexed CRISPR-Cas system targeting nine specific loci in the ASFV genome. This innovative approach successfully inhibits ASFV replication in vitro, and we have successfully engineered pig strains to express this anti-ASFV CRISPR-Cas system constitutively. Despite not observing survival advantages in these transgenic pigs upon ASFV challenges, we did note a delay in infection in some cases. To the best of our knowledge, this study constitutes the first example of a germline-edited animal with an anti-virus CRISPR-Cas system. These findings contribute to the advancement of future anti-viral strategies and the optimization of viral immunity technologies.
Keywords: African swine fever virus; CRISPR; agriculture; pig; xenotransplantation.
Conflict of interest statement
Y.Y., L.X., H.D., Y.Z., X.F., X.H., Z.T., L.S., Y.G., G.M., J.H., and L.Y. are employed by Qihan Bio Inc. G.M.C. is the cofounder and scientific advisor of Qihan Bio Inc.
Figures

Comment in
-
Multiplexed CRISPR-Cas system targeting ASFV genes in vivo: solution lies within.Microbiol Spectr. 2024 Sep 3;12(9):e0071424. doi: 10.1128/spectrum.00714-24. Epub 2024 Aug 7. Microbiol Spectr. 2024. PMID: 39109857 Free PMC article.
References
-
- African swine fever (ASF) – situation report 10. 2022. World organisation for animal health
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

