Pulmonary Vein Isolation With or Without Left Atrial Appendage Ligation in Atrial Fibrillation: The aMAZE Randomized Clinical Trial
- PMID: 38563835
- PMCID: PMC10988350
- DOI: 10.1001/jama.2024.3026
Pulmonary Vein Isolation With or Without Left Atrial Appendage Ligation in Atrial Fibrillation: The aMAZE Randomized Clinical Trial
Abstract
Importance: Left atrial appendage elimination may improve catheter ablation outcomes for atrial fibrillation.
Objective: To assess the safety and effectiveness of percutaneous left atrial appendage ligation adjunctive to catheter pulmonary vein isolation for nonparoxysmal atrial fibrillation.
Design, setting, and participants: This multicenter, prospective, open-label, randomized clinical trial evaluated the safety and effectiveness of percutaneous left atrial appendage ligation adjunctive to planned pulmonary vein isolation for nonparoxysmal atrial fibrillation present for less than 3 years. Eligible patients were randomized in a 2:1 ratio to undergo left atrial appendage ligation and pulmonary vein isolation or pulmonary vein isolation alone. Use of a 2:1 randomization ratio was intended to provide more device experience and safety data. Patients were enrolled from October 2015 to December 2019 at 53 US sites, with the final follow-up visit on April 21, 2021.
Interventions: Left atrial appendage ligation plus pulmonary vein isolation compared with pulmonary vein isolation alone.
Main outcomes and measures: A bayesian adaptive analysis was used for primary end points. Primary effectiveness was freedom from documented atrial arrythmias of greater than 30 seconds duration 12 months after undergoing pulmonary vein isolation. Rhythm was assessed by Holter monitoring at 6 and 12 months after pulmonary vein isolation, symptomatic event monitoring, or any electrocardiographic tracing obtained through 12 months after pulmonary vein isolation. Primary safety was a composite of predefined serious adverse events compared with a prespecified 10% performance goal 30 days after the procedure. Left atrial appendage closure was evaluated through 12 months after pulmonary vein isolation.
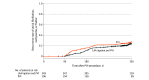
Results: Overall, 404 patients were randomized to undergo left atrial appendage ligation plus pulmonary vein isolation and 206 were randomized to undergo pulmonary vein isolation alone. Primary effectiveness was 64.3% with left atrial appendage ligation and pulmonary vein isolation and 59.9% with pulmonary vein isolation only (difference, 4.3% [bayesian 95% credible interval, -4.2% to 13.2%]; posterior superiority probability, 0.835), which did not meet the statistical criterion to establish superiority (0.977). Primary safety was met, with a 30-day serious adverse event rate of 3.4% (bayesian 95% credible interval, 2.0% to 5.0%; posterior probability, 1.0) which was less than the prespecified threshold of 10%. At 12 months after pulmonary vein isolation, complete left atrial appendage closure (0 mm residual communication) was observed in 84% of patients and less than or equal to 5 mm residual communication was observed in 99% of patients.
Conclusions and relevance: Percutaneous left atrial appendage ligation adjunctive to pulmonary vein isolation did not meet prespecified efficacy criteria for freedom from atrial arrhythmias at 12 months compared with pulmonary vein isolation alone for patients with nonparoxysmal atrial fibrillation, but met prespecified safety criteria and demonstrated high rates of closure at 12 months.
Trial registration: ClinicalTrials.gov Identifier: NCT02513797.
Conflict of interest statement
Figures

Comment in
-
Pulmonary Vein Isolation and Left Atrial Appendage Ligation in Atrial Fibrillation.JAMA. 2024 Sep 3;332(9):759. doi: 10.1001/jama.2024.13523. JAMA. 2024. PMID: 39115818 No abstract available.
References
-
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149(1):1-156. doi: 10.1161/CIR.0000000000001193 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

