Gyrase and Topoisomerase IV: Recycling Old Targets for New Antibacterials to Combat Fluoroquinolone Resistance
- PMID: 38564341
- PMCID: PMC11019561
- DOI: 10.1021/acsinfecdis.4c00128
Gyrase and Topoisomerase IV: Recycling Old Targets for New Antibacterials to Combat Fluoroquinolone Resistance
Abstract
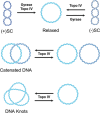
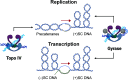
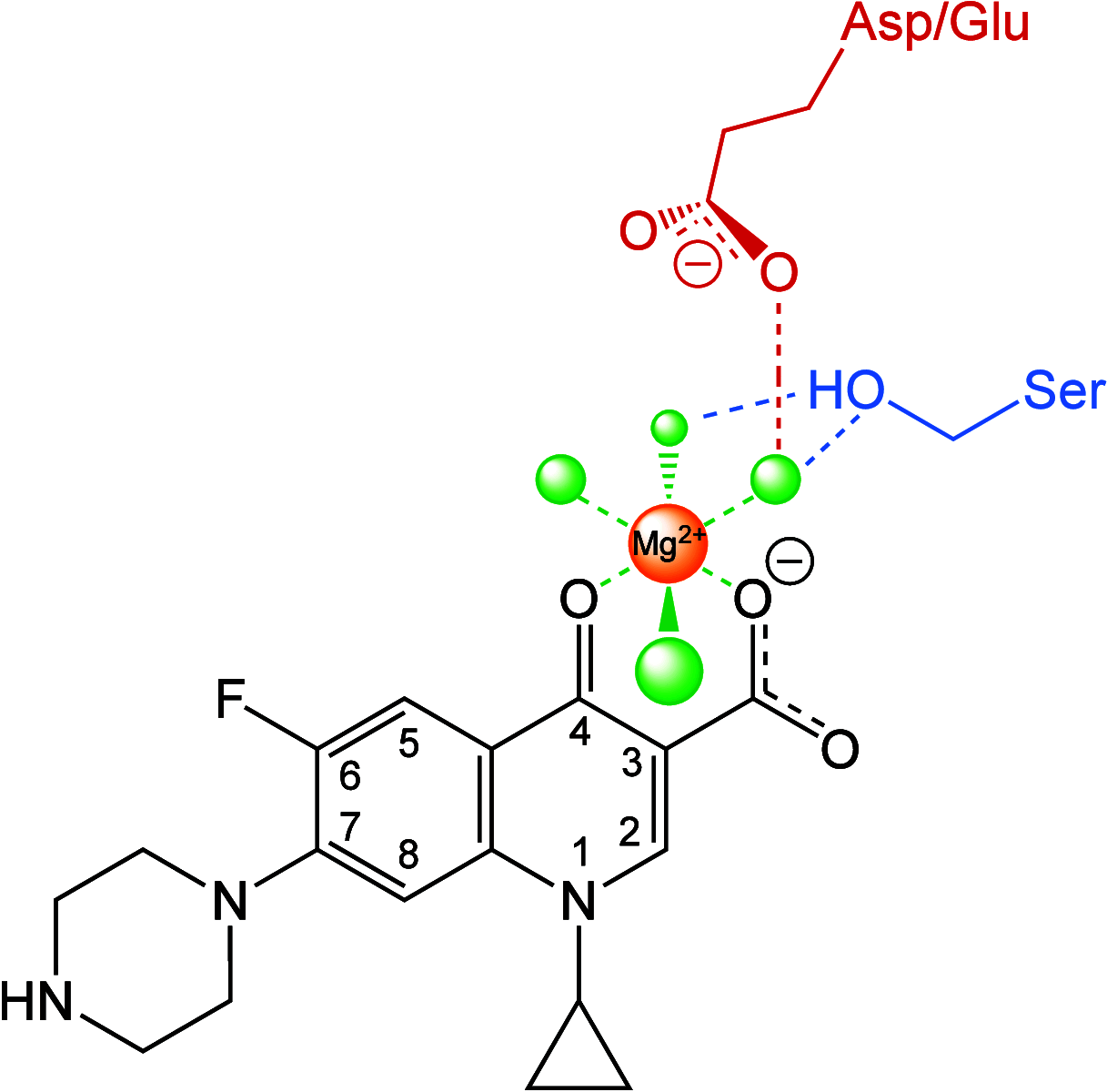
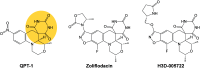
Beyond their requisite functions in many critical DNA processes, the bacterial type II topoisomerases, gyrase and topoisomerase IV, are the targets of fluoroquinolone antibacterials. These drugs act by stabilizing gyrase/topoisomerase IV-generated DNA strand breaks and by robbing the cell of the catalytic activities of these essential enzymes. Since their clinical approval in the mid-1980s, fluoroquinolones have been used to treat a broad spectrum of infectious diseases and are listed among the five "highest priority" critically important antimicrobial classes by the World Health Organization. Unfortunately, the widespread use of fluoroquinolones has been accompanied by a rise in target-mediated resistance caused by specific mutations in gyrase and topoisomerase IV, which has curtailed the medical efficacy of this drug class. As a result, efforts are underway to identify novel antibacterials that target the bacterial type II topoisomerases. Several new classes of gyrase/topoisomerase IV-targeted antibacterials have emerged, including novel bacterial topoisomerase inhibitors, Mycobacterium tuberculosis gyrase inhibitors, triazaacenaphthylenes, spiropyrimidinetriones, and thiophenes. Phase III clinical trials that utilized two members of these classes, gepotidacin (triazaacenaphthylene) and zoliflodacin (spiropyrimidinetrione), have been completed with positive outcomes, underscoring the potential of these compounds to become the first new classes of antibacterials introduced into the clinic in decades. Because gyrase and topoisomerase IV are validated targets for established and emerging antibacterials, this review will describe the catalytic mechanism and cellular activities of the bacterial type II topoisomerases, their interactions with fluoroquinolones, the mechanism of target-mediated fluoroquinolone resistance, and the actions of novel antibacterials against wild-type and fluoroquinolone-resistant gyrase and topoisomerase IV.
Keywords: fluoroquinolone resistance; gyrase; novel bacterial topoisomerase inhibitors; spiropyrimidinetriones; topoisomerase IV; triazaacenaphthylenes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Gibson E. G.; Ashley R. E.; Kerns R. J.; Osheroff N.. Fluoroquinolone interactions with bacterial type II topoisomerases and target-mediated drug resistance. In Antimicrobial Resistance in the 21st Century, 2nd ed.; Drlica K., Shlaes D., Fong I. W., Eds.; Springer Nature, 2018; pp 507–529.
-
- Hiasa H.DNA topoisomerases as targets for antibacterial agents. In DNA Topoisomerases, Drolet M., Ed.; Methods in Molecular Biology, Vol. 1703; Humana Press, 2018; pp 47–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

