Pigeon pea crop stage strongly influences plant susceptibility to Helicoverpa armigera (Lepidoptera: Noctuidae)
- PMID: 38564410
- PMCID: PMC11163456
- DOI: 10.1093/jee/toae050
Pigeon pea crop stage strongly influences plant susceptibility to Helicoverpa armigera (Lepidoptera: Noctuidae)
Abstract
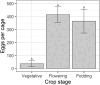
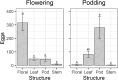
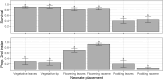
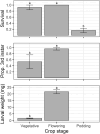
Helicoverpa armigera Hübner (Lepidoptera: Noctuidae; Hübner) is the major insect pest of pigeon pea [Cajanus cajan; Fabales: Fabaceae; (L.) Millspaugh] worldwide. Research to develop pest management strategies for H. armigera in pigeon pea has focused heavily on developing less susceptible cultivars, with limited practical success. We examined how pigeon pea crop stage influences plant susceptibility to H. armigera using a combination of glasshouse and laboratory experiments. Plant phenology significantly affected oviposition with moths laying more eggs on flowering and podding plants but only a few on vegetative plants. Larval survival was greatest on flowering and vegetative plants, wherein larvae mostly chose to feed inside flowers on flowering plants and on the adaxial surface of expanding leaves on vegetative plants. Larval survival was poor on podding plants despite moths laying many eggs on plants of this stage. When left to feed without restriction on plants for 7 days, larvae feeding on flowering plants were >10 times the weight of larvae feeding on plants of other phenological stages. On whole plants, unrestricted larvae preferred to feed on pigeon pea flowers and on expanding leaves, but in no-choice Petri dish assays H. armigera larvae could feed and survive on all pigeon pea reproductive structures. Our results show that crop stage and the availability of flowers strongly influence pigeon pea susceptibility to H. armigera. An increased understanding of H. armigera-pigeon pea ecology will be useful in guiding the development of resistant varieties and other management tactics.
Keywords: herbivory; host-plant resistance; phenology; preference–performance hypothesis.
© The Author(s) 2024. Published by Oxford University Press on behalf of Entomological Society of America.
Figures




Similar articles
-
Helicoverpa armigera preference and performance on three cultivars of short-duration pigeonpea (Cajanus cajan): the importance of whole plant assays.Pest Manag Sci. 2023 Feb;79(2):627-637. doi: 10.1002/ps.7230. Epub 2022 Oct 27. Pest Manag Sci. 2023. PMID: 36222835 Free PMC article.
-
The movement and distribution of Helicoverpa armigera (Hübner) larvae on pea plants is affected by egg placement and flowering.Bull Entomol Res. 2010 Oct;100(5):591-8. doi: 10.1017/S0007485309990654. Epub 2010 May 27. Bull Entomol Res. 2010. PMID: 20504381
-
Comparative analysis of herbivory responsive miRNAs to delineate pod borer (Helicoverpa armigera) resistance mechanisms in Cajanus cajan and its wild relative Cajanus scarabaeoides.Plant Cell Rep. 2022 Apr;41(4):1147-1161. doi: 10.1007/s00299-022-02842-5. Epub 2022 Apr 2. Plant Cell Rep. 2022. PMID: 35366099
-
Understanding heliothine (Lepidoptera: Heliothinae) pests: what is a host plant?J Econ Entomol. 2014 Jun;107(3):881-96. doi: 10.1603/ec14036. J Econ Entomol. 2014. PMID: 25026644 Review.
-
Neuronal architecture and functional mapping of the taste center of larval Helicoverpa armigera (Lepidoptera: Noctuidae).Insect Sci. 2022 Jun;29(3):730-748. doi: 10.1111/1744-7917.12965. Epub 2021 Oct 15. Insect Sci. 2022. PMID: 34427391 Review.
Cited by
-
Ontogenetic Changes in the Feeding Behaviour of Helicoverpa armigera Larvae on Pigeonpea (Cajanus cajan) Flowers and Pods.Plants (Basel). 2024 Feb 29;13(5):696. doi: 10.3390/plants13050696. Plants (Basel). 2024. PMID: 38475544 Free PMC article.
References
-
- Ang GC, Silva R, Maxwell SL, Zalucki MP, Furlong MJ.. Contrary effects of leaf position and identity on oviposition and larval feeding patterns of the diamondback moth. Entomol Exp Appl. 2014:151(1):86–96. 10.1111/eea.12172 - DOI
-
- Borges M, Michereff M, Laumann R, Santana G, Castro B, Silva C, Blassioli-Moraes M.. Influence of pigeon pea (Cajanus cajan) on oviposition behaviour of Diceraeus melacanthus stink bug, an important pest of soybean and maize crops in South America. Arthropod Plant Interact. 2023:17(1):77–89.
-
- Cotter S, Edwards O.. Quantitative genetics of preference and performance on chickpeas in the noctuid moth, Helicoverpa armigera. Hered. 2006:96(5):396–402. - PubMed
-
- Cribb BW, Hanan J, Zalucki MP, Perkins LE.. Effects of plant micro-environment on movement of Helicoverpa armigera (Hübner) larvae and the relationship to a hierarchy of stimuli. Arthropod Plant Interact. 2010:4(3):165–173. 10.1007/s11829-010-9097-0 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

