Iron Oxide Nanoparticles Inhibit Tumor Progression and Suppress Lung Metastases in Mouse Models of Breast Cancer
- PMID: 38564478
- PMCID: PMC11025112
- DOI: 10.1021/acsnano.3c12064
Iron Oxide Nanoparticles Inhibit Tumor Progression and Suppress Lung Metastases in Mouse Models of Breast Cancer
Abstract
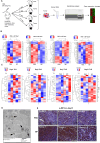
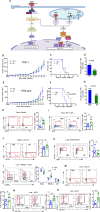
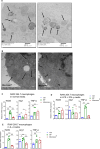
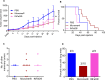
Systemic exposure to starch-coated iron oxide nanoparticles (IONPs) can stimulate antitumor T cell responses, even when little IONP is retained within the tumor. Here, we demonstrate in mouse models of metastatic breast cancer that IONPs can alter the host immune landscape, leading to systemic immune-mediated disease suppression. We report that a single intravenous injection of IONPs can inhibit primary tumor growth, suppress metastases, and extend survival. Gene expression analysis revealed the activation of Toll-like receptor (TLR) pathways involving signaling via Toll/Interleukin-1 receptor domain-containing adaptor-inducing IFN-β (TRIF), a TLR pathway adaptor protein. Requisite participation of TRIF in suppressing tumor progression was demonstrated with histopathologic evidence of upregulated IFN-regulatory factor 3 (IRF3), a downstream protein, and confirmed in a TRIF knockout syngeneic mouse model of metastatic breast cancer. Neither starch-coated polystyrene nanoparticles lacking iron, nor iron-containing dextran-coated parenteral iron replacement agent, induced significant antitumor effects, suggesting a dependence on the type of IONP formulation. Analysis of multiple independent clinical databases supports a hypothesis that upregulation of TLR3 and IRF3 correlates with increased overall survival among breast cancer patients. Taken together, these data support a compelling rationale to re-examine IONP formulations as harboring anticancer immune (nano)adjuvant properties to generate a therapeutic benefit without requiring uptake by cancer cells.
Keywords: Breast cancer metastasis; Iron oxide nanoparticles; T cell signaling; Toll-like receptors; Toll/interleukin-1 receptor-domain-containing adaptor-inducing interferon-β (TRIF).
Conflict of interest statement
The authors declare the following competing financial interest(s): R.I. is an inventor on several issued and pending patents. All patents are assigned to Johns Hopkins University or Aduro Biotech, Inc. All other authors declare no competing interests.
Figures




Similar articles
-
Angiotensin II-induced hypertension and cardiac hypertrophy are differentially mediated by TLR3- and TLR4-dependent pathways.Am J Physiol Heart Circ Physiol. 2019 May 1;316(5):H1027-H1038. doi: 10.1152/ajpheart.00697.2018. Epub 2019 Feb 22. Am J Physiol Heart Circ Physiol. 2019. PMID: 30793936 Free PMC article.
-
Toll-like receptor 3 and 4 signalling through the TRIF and TRAM adaptors in haematopoietic cells promotes atherosclerosis.Cardiovasc Res. 2013 Jul 15;99(2):364-73. doi: 10.1093/cvr/cvt033. Epub 2013 Feb 14. Cardiovasc Res. 2013. PMID: 23417039
-
Role of Adaptor Protein Toll-Like Interleukin Domain Containing Adaptor Inducing Interferon β in Toll-Like Receptor 3- and 4-Mediated Regulation of Hepatic Drug Metabolizing Enzyme and Transporter Genes.Drug Metab Dispos. 2016 Jan;44(1):61-7. doi: 10.1124/dmd.115.066761. Epub 2015 Oct 15. Drug Metab Dispos. 2016. PMID: 26470915 Free PMC article.
-
Modulation of Toll-interleukin 1 receptor mediated signaling.J Mol Med (Berl). 2005 Apr;83(4):258-66. doi: 10.1007/s00109-004-0622-4. Epub 2005 Jan 21. J Mol Med (Berl). 2005. PMID: 15662540 Review.
-
Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses.J Zhejiang Univ Sci B. 2021 Aug 15;22(8):609-632. doi: 10.1631/jzus.B2000808. J Zhejiang Univ Sci B. 2021. PMID: 34414698 Free PMC article. Review.
Cited by
-
Toll-Like Receptors in the Immunotherapy Era: Dual-Edged Swords of Tumor Immunity and Clinical Translation.MedComm (2020). 2025 Jul 27;6(8):e70308. doi: 10.1002/mco2.70308. eCollection 2025 Aug. MedComm (2020). 2025. PMID: 40727252 Free PMC article. Review.
-
A comprehensive review of using nanomaterials in cancer immunotherapy: Pros and Cons of clinical usage.3 Biotech. 2025 Jul;15(7):205. doi: 10.1007/s13205-025-04362-x. Epub 2025 Jun 9. 3 Biotech. 2025. PMID: 40502964 Review.
-
Fe3O4 nanoparticles containing gambogic acid inhibit metastasis in colorectal cancer via the RORB/EMILIN1 axis.Cell Adh Migr. 2024 Dec;18(1):38-53. doi: 10.1080/19336918.2024.2427585. Epub 2024 Nov 13. Cell Adh Migr. 2024. PMID: 39533963 Free PMC article.
-
Engineered iron oxide nanoplatforms: reprogramming immunosuppressive niches for precision cancer theranostics.Mol Cancer. 2025 Sep 1;24(1):225. doi: 10.1186/s12943-025-02443-2. Mol Cancer. 2025. PMID: 40887613 Free PMC article. Review.
-
Polysaccharide nanoparticles as potential immune adjuvants: Mechanism and function.Acta Pharm Sin B. 2025 Apr;15(4):1796-1815. doi: 10.1016/j.apsb.2025.03.006. Epub 2025 Mar 7. Acta Pharm Sin B. 2025. PMID: 40486863 Free PMC article. Review.
References
-
- Allemani; Matsuda C.; Di Carlo T.; Harewood V.; Matz R.; Nikšić M.; Bonaventure M.; Valkov A.; Johnson M.; Estève C. J.; Ogunbiyi J.; Azevedo O. J.; Silva E.; Chen W. Q.; Eser S.; Engholm G.; Stiller C. A.; Monnereau A.; Woods R. R.; Visser O.; Lim G. H.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37513025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391 (10125), 1023–1075. 10.1016/S0140-6736(17)33326-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

