Combination Treatment with Verinurad and Allopurinol in CKD: A Randomized Placebo and Active Controlled Trial
- PMID: 38564654
- PMCID: PMC11149044
- DOI: 10.1681/ASN.0000000000000326
Combination Treatment with Verinurad and Allopurinol in CKD: A Randomized Placebo and Active Controlled Trial
Abstract
Key Points:
The SAPPHIRE trial was designed to assess albuminuria-lowering effects of the urate transporter 1 inhibitor verinurad combined with allopurinol in patients with CKD.
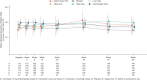
Verinurad 3, 7.5, and 12 mg in combination with allopurinol 300 mg did not reduce albuminuria during 34 weeks treatment compared with allopurinol alone or placebo.
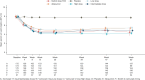
Verinurad/allopurinol combination dose-dependently reduced serum urate concentrations compared with placebo.
Background: Hyperuricemia is associated with elevated risks of cardiovascular and chronic kidney disease (CKD). Since inhibition of urate transporter 1 has been suggested to be potentially nephroprotective, we performed a phase 2b study to assess albuminuria-lowering effects of the urate transporter 1 inhibitor verinurad combined with the xanthine oxidase inhibitor allopurinol in patients with CKD and hyperuricemia.
Methods: In this randomized placebo and active controlled trial, we enrolled participants with serum urate concentrations ≥6.0 mg/dl, eGFR ≥25 ml/min per 1.73 m2, and a urinary albumin-creatinine ratio (UACR) 30–5000 mg/g to one of five treatment arms: placebo, placebo+allopurinol 300 mg/day, verinurad 3 mg+allopurinol 300 mg/day, verinurad 7.5 mg+allopurinol 300 mg/day, or verinurad 12 mg+allopurinol 300 mg/day in a 1:1:1:1:1 ratio. The primary end point was the change in UACR from baseline to 34 weeks. Secondary end points were changes from baseline in UACR at week 60 and changes in serum urate and eGFR at weeks 34 and 60.
Results: Between August 2019 and November 2021, 861 adults with CKD (mean age 65 years, 33.0% female, mean eGFR 48 ml/min per 1.73 m2, median UACR 217 mg/g) were enrolled. At 34 weeks, the geometric mean percentage change in UACR from baseline did not differ among treatment groups (16.7%, 95% confidence interval [CI], −0.6 to 37.1 in the 3 mg group, 15.0% [95% CI, −1.85 to 34.6] in the 7.5 mg group, 14.0% [95% CI, −3.4 to 34.4] in the 12 mg group versus 9.9% [95% CI, −6.6 to 29.4] in the allopurinol group, and 37.3% [95% CI, 16.6 to 61.8] in the placebo group). UACR and eGFR change from baseline did not differ among treatment groups after 60 weeks. Verinurad/allopurinol combination dose-dependently reduced serum urate concentrations compared with placebo. The proportion of patients with adverse events and serious adverse events was balanced among treatment groups.
Conclusions: Verinurad in combination with allopurinol did not decrease UACR or eGFR decline, but further reduced serum urate compared with allopurinol alone or placebo.
Clinical Trial registry name and registration number::
SAPPHIRE Trial registration number,
Trial registration: ClinicalTrials.gov NCT03990363 NCT03036150.
Conflict of interest statement
M. Bjursell reports employment with AstraZeneca and ownership interest (shares) in AstraZeneca, Medivir, and Swedish Orphan Biovitrum. O. Eklund reports employment with and ownership interest in AstraZeneca. H.J.L. Heerspink reports ongoing consultancy agreements with AstraZeneca, Bayer, BioChryst, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Eli Lilly, Gilead, Janssen, Merck, Novartis, Novo Nordisk, and Travere Pharmaceuticals; research funding from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk, and Janssen research support (grant funding directed to employer); lecture fees from AstraZeneca and Novo Nordisk; and speakers bureau for AstraZeneca. L.A. Inker reports consultancy for Diamtrix; consulting agreements to Tufts Medical Center with Tricida; funding to institute, Tufts Medical Center, for research and contracts with the National Institutes of Health, National Kidney Foundation, Chinook, Omeros, and Reata Pharmaceuticals; consulting agreements with Tricida Inc.; advisory or leadership role for Alport Foundation Medical Advisory Council and National Kidney Foundation Scientific Advisory Board; and other interests or relationships as an American Society of Nephrology member and National Kidney Foundation member. N. Jongs reports serving on speakers bureau for AstraZeneca and travel support from AstraZeneca. N. Maklad reports employment with AstraZeneca. V. Perkovic reports consultancy for AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Travere, Tricida, and UpToDate; ownership interest in George Clinical; research funding from AstraZeneca, Bayer, Chinook, Gilead, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Otsuka, Travere, and Tricida; honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Travere, Tricida, and UpToDate; honoraria for Steering Committee roles, scientific presentations, and/or advisory board attendance from Abbvie, Amgen, AstraZeneca, Baxter, Bayer, Boehringer Ingelheim, Chinook, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Pfizer, Pharmalink, Reata, Relypsa, Roche, Sanofi, Servier, Travere, and Tricida; advisory or leadership roles on Steering Committees for Bayer, Chinook, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Otsuka, Pfizer, and Travere; and advisory or leadership role as Board Director for St Vincents Health Australia, George Clinical. S. Perl reports employment with, ownership interest in, and other interests or relationships with AstraZeneca. T. Rikte reports employment with AstraZeneca and ownership interest in Novo Nordisk A/S, Denmark and Telia, Sweden. T. Rikte's partner is a nurse (anesthesia) at Skånes Universitetssjukhus (“University hospital of Scania”), Malmö, Sweden. C.D. Sjostrom reports employment with and ownership interest in AstraZeneca LP. A.G. Stack reports consultancy for AstraZeneca, Menarini, and Vifor Pharma; educational grant from Vifor Pharma and research funding from AstraZeneca; honoraria from AstraZeneca, Menarini, and Vifor; patents or royalties from Preserva Medical; role on Editorial Board of
Figures





References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

