Relationship of plasma 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid concentration with OATP1B activity in patients with chronic kidney disease
- PMID: 38564661
- PMCID: PMC10844757
- DOI: 10.1111/cts.13731
Relationship of plasma 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid concentration with OATP1B activity in patients with chronic kidney disease
Abstract
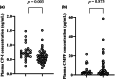
Organic anion-transporting polypeptides (OATP)1B are drug transporters mainly expressed in the sinusoidal membrane. Many studies have suggested that OATP1B activity is affected by genetic factor, the uremic toxin 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF), and inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Coproporphyrin-I (CP-I) is spotlighted as a highly accurate endogenous substrate of OATP1B. We previously reported a positive correlation between plasma CMPF and CP-I concentrations in patients with chronic kidney disease (CKD). The present study evaluated the impact of genetic polymorphisms, CMPF, IL-6, TNF-α, and estimated glomerular filtration rate (eGFR) on individual differences in OATP1B activity in patients with CKD. Seventy-three patients with CKD who received kidney transplant at least 3 months earlier were analyzed. Plasma CP-I concentration was higher in OATP1B1*15 carriers than in non-carriers. In all patients, CP-I did not correlate significantly with CMPF, IL-6, TNF-α, or eGFR. However, when the dataset was cut off at CMPF concentration of 8 and 7 μg/mL, 4 μg/mL, 3 μg/mL or 2 μg/mL, CMPF correlated positively with CP-I, and correlation coefficient tended to be higher as plasma CMPF concentration was lower. In conclusion, OATP1B1*15 impacted OATP1B activity in patients with CKD, but IL-6 and TNF-α did not. However, the impact of CMPF on OATP1B activity was limited to low CMPF concentrations, and the effect could be saturated at high concentrations. When prescribing an OATP1B substrate drug for patients with CKD, the OATP1B1*15 carrier status and plasma CMPF concentration may need to be considered to decide the dose regimen.
© 2024 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures



References
-
- Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82:726‐733. - PubMed
-
- Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008;36:2014‐2023. - PubMed
-
- Menzel K, Kothare P, McCrea JB, Chu X, Kropeit D. Absorption, metabolism, distribution, and excretion of letermovir. Curr Drug Metab. 2021;22:784‐794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

