Αnti-prion effects of anthocyanins
- PMID: 38565068
- PMCID: PMC10990977
- DOI: 10.1016/j.redox.2024.103133
Αnti-prion effects of anthocyanins
Abstract
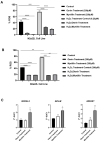
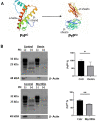
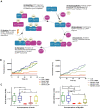
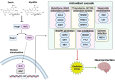
Prion diseases, also known as Transmissible Spongiform Encephalopathies (TSEs), are protein-based neurodegenerative disorders (NDs) affecting humans and animals. They are characterized by the conformational conversion of the normal cellular prion protein, PrPC, into the pathogenic isoform, PrPSc. Prion diseases are invariably fatal and despite ongoing research, no effective prophylactic or therapeutic avenues are currently available. Anthocyanins (ACNs) are unique flavonoid compounds and interest in their use as potential neuroprotective and/or therapeutic agents against NDs, has increased significantly in recent years. Therefore, we investigated the potential anti-oxidant and anti-prion effects of Oenin and Myrtillin, two of the most common anthocyanins, using the most accepted in the field overexpressing PrPScin vitro model and a cell free protein aggregation model. Our results, indicate both anthocyanins as strong anti-oxidant compounds, upregulating the expression of genes involved in the anti-oxidant response, and reducing the levels of Reactive Oxygen Species (ROS), produced due to pathogenic prion infection, through the activation of the Keap1-Nrf2 pathway. Importantly, they showcased remarkable anti-prion potential, as they not only caused the clearance of pathogenic PrPSc aggregates, but also completely inhibited the formation of PrPSc fibrils in the Cerebrospinal Fluid (CSF) of patients with Creutzfeldt-Jakob disease (CJD). Therefore, Oenin and Myrtillin possess pleiotropic effects, suggesting their potential use as promising preventive and/or therapeutic agents in prion diseases and possibly in the spectrum of neurodegenerative proteinopathies.
Keywords: Anthocyanins; Anti-oxidant; Neuroprotection; PrP(Sc); Prion; Proteinopathies.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

