TRPM2 and CaMKII Signaling Drives Excessive GABAergic Synaptic Inhibition Following Ischemia
- PMID: 38565288
- PMCID: PMC11079974
- DOI: 10.1523/JNEUROSCI.1762-23.2024
TRPM2 and CaMKII Signaling Drives Excessive GABAergic Synaptic Inhibition Following Ischemia
Abstract
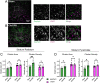
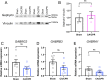
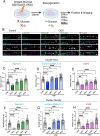
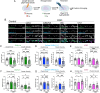
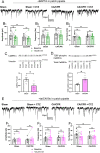
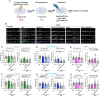
Excitotoxicity and the concurrent loss of inhibition are well-defined mechanisms driving acute elevation in excitatory/inhibitory (E/I) balance and neuronal cell death following an ischemic insult to the brain. Despite the high prevalence of long-term disability in survivors of global cerebral ischemia (GCI) as a consequence of cardiac arrest, it remains unclear whether E/I imbalance persists beyond the acute phase and negatively affects functional recovery. We previously demonstrated sustained impairment of long-term potentiation (LTP) in hippocampal CA1 neurons correlating with deficits in learning and memory tasks in a murine model of cardiac arrest/cardiopulmonary resuscitation (CA/CPR). Here, we use CA/CPR and an in vitro ischemia model to elucidate mechanisms by which E/I imbalance contributes to ongoing hippocampal dysfunction in male mice. We reveal increased postsynaptic GABAA receptor (GABAAR) clustering and function in the CA1 region of the hippocampus that reduces the E/I ratio. Importantly, reduced GABAAR clustering observed in the first 24 h rebounds to an elevation of GABAergic clustering by 3 d postischemia. This increase in GABAergic inhibition required activation of the Ca2+-permeable ion channel transient receptor potential melastatin-2 (TRPM2), previously implicated in persistent LTP and memory deficits following CA/CPR. Furthermore, we find Ca2+-signaling, likely downstream of TRPM2 activation, upregulates Ca2+/calmodulin-dependent protein kinase II (CaMKII) activity, thereby driving the elevation of postsynaptic inhibitory function. Thus, we propose a novel mechanism by which inhibitory synaptic strength is upregulated in the context of ischemia and identify TRPM2 and CaMKII as potential pharmacological targets to restore perturbed synaptic plasticity and ameliorate cognitive function.
Keywords: E/I balance; GABAA receptors; TRPM2; cardiac arrest; global cerebral ischemia; inhibitory synapse.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
