Low-frequency magnetic field therapy for glioblastoma: Current advances, mechanisms, challenges and future perspectives
- PMID: 38565404
- PMCID: PMC11954840
- DOI: 10.1016/j.jare.2024.03.024
Low-frequency magnetic field therapy for glioblastoma: Current advances, mechanisms, challenges and future perspectives
Abstract
Background: Glioblastoma (GBM) is the most common malignant tumour of the central nervous system. Despite recent advances in multimodal GBM therapy incorporating surgery, radiotherapy, systemic therapy (chemotherapy, targeted therapy), and supportive care, the overall survival (OS) remains poor, and long-term survival is rare. Currently, the primary obstacles hindering the effectiveness of GBM treatment are still the blood-brain barrier and tumor heterogeneity. In light of its substantial advantages over conventional therapies, such as strong penetrative ability and minimal side effects, low-frequency magnetic fields (LF-MFs) therapy has gradually caught the attention of scientists.
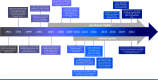
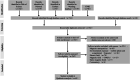
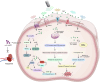
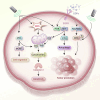
Aim of review: In this review, we shed the light on the current status of applying LF-MFs in the treatment of GBM. We specifically emphasize our current understanding of the mechanisms by which LF-MFs mediate anticancer effects and the challenges faced by LF-MFs in treating GBM cells. Furthermore, we discuss the prospective applications of magnetic field therapy in the future treatment of GBM. Key scientific concepts of review: The review explores the current progress on the use of LF-MFs in the treatment of GBM with a special focus on the potential underlying mechanisms of LF-MFs in anticancer effects. Additionally, we also discussed the complex magnetic field features and biological characteristics related to magnetic bioeffects. Finally, we proposed a promising magnetic field treatment strategy for future applications in GBM therapy.
Keywords: Antitumour; Glioblastoma; Low-frequency magnetic fields; Molecular mechanism.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Tan A.C., Ashley D.M., López G.Y., Malinzak M., Friedman H.S., Khasraw M. Management of glioblastoma: state of the art and future directions. CA Cancer J Clin. 2020;70:299–312. - PubMed
-
- Nicholson J.G., Fine H.A. Diffuse glioma heterogeneity and its therapeutic implications. Cancer Discov. 2021;11:575–590. - PubMed
-
- Yao J., Feng J., Chen J. External-stimuli responsive systems for cancer theranostic. Asian J Pharm Sci. 2016;11:585–595.
-
- Ulasov I.V., Foster H., Butters M., Yoon J.G., Ozawa T., Nicolaides T., et al. Precision knockdown of EGFR gene expression using radio frequency electromagnetic energy. J Neurooncol. 2017;133:257–264. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

