Umpolung reactivity of strained C-C σ-bonds without transition-metal catalysis
- PMID: 38565533
- PMCID: PMC10987681
- DOI: 10.1038/s41467-024-47169-9
Umpolung reactivity of strained C-C σ-bonds without transition-metal catalysis
Abstract
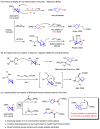
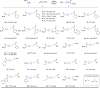
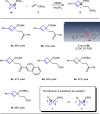
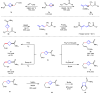
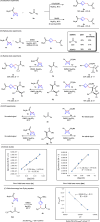
Umpolung is an old and important concept in organic chemistry, which significantly expands the chemical space and provides unique structures. While, previous research focused on carbonyls or imine derivatives, the umpolung reactivity of polarized C-C σ-bonds still needs to explore. Herein, we report an umpolung reaction of bicyclo[1.1.0]butanes (BCBs) with electron-deficient alkenes to construct the C(sp3)-C(sp3) bond at the electrophilic position of C-C σ-bonds in BCBs without any transition-metal catalysis. Specifically, this transformation relies on the strain-release driven bridging σ-bonds in bicyclo[1.1.0]butanes (BCBs), which are emerged as ene components, providing an efficient and straightforward synthesis route of various functionalized cyclobutenes and conjugated dienes, respectively. The synthetic utilities of this protocol are performed by several transformations. Preliminary mechanistic studies including density functional theory (DFT) calculation support the concerted Alder-ene type process of C-C σ-bond cleavage with hydrogen transfer. This work extends the umpolung reaction to C-C σ-bonds and provides high-value structural motifs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Stereoselective Alder-Ene Reactions of Bicyclo[1.1.0]butanes: Facile Synthesis of Cyclopropyl- and Aryl-Substituted Cyclobutenes.J Am Chem Soc. 2024 Jan 10;146(1):1196-1203. doi: 10.1021/jacs.3c13080. Epub 2023 Dec 29. J Am Chem Soc. 2024. PMID: 38157245 Free PMC article.
-
Ring-strain-enabled reaction discovery: new heterocycles from bicyclo[1.1.0]butanes.Acc Chem Res. 2015 Apr 21;48(4):1149-58. doi: 10.1021/ar500437h. Epub 2015 Mar 16. Acc Chem Res. 2015. PMID: 25775119
-
DFT Insight into a Strain-Release Mechanism in Bicyclo[1.1.0]butanes via Concerted Activation of Central and Lateral C-C Bonds with Rh(III) Catalysis.Inorg Chem. 2024 May 13;63(19):8879-8888. doi: 10.1021/acs.inorgchem.4c00800. Epub 2024 Apr 27. Inorg Chem. 2024. PMID: 38676642
-
Transition metal-carboryne complexes: synthesis, bonding, and reactivity.Acc Chem Res. 2011 Apr 19;44(4):299-309. doi: 10.1021/ar100156f. Epub 2011 Mar 11. Acc Chem Res. 2011. PMID: 21395260 Review.
-
Metal-Free Catalysis in C-C Single-Bond Cleavage: Achievements and Prospects.Top Curr Chem (Cham). 2022 Aug 11;380(5):38. doi: 10.1007/s41061-022-00393-7. Top Curr Chem (Cham). 2022. PMID: 35951267 Review.
Cited by
-
(3 + 2)-Cycloaddition of bicyclobutanes and thioketones: access to 2-thiabicyclo[2.1.1]hexanes without the use of catalysts or light.Chem Sci. 2025 Apr 2;16(19):8588-8593. doi: 10.1039/d5sc00125k. eCollection 2025 May 14. Chem Sci. 2025. PMID: 40321184 Free PMC article.
-
Photoinduced cobaloxime catalysis enabled dehydrogenative C2-phosphinylation of bicyclo[1.1.0]butanes to access phosphorylated cyclobutenes.RSC Adv. 2025 Jul 10;15(29):24053-24057. doi: 10.1039/d5ra03697f. eCollection 2025 Jul 4. RSC Adv. 2025. PMID: 40642465 Free PMC article.
-
Mechanistic Pathways in Cyanide-Mediated Benzoin Condensation: A Comprehensive Electron Localisation Function (ELF) and Catastrophe Theory Analysis of the Umpolung Reaction.Molecules. 2025 Jan 17;30(2):378. doi: 10.3390/molecules30020378. Molecules. 2025. PMID: 39860246 Free PMC article.
-
A general leaving group assisted strategy for synthesis of pentafluorosulfanyl allylic compounds.Sci Adv. 2025 Jul 25;11(30):eadw8408. doi: 10.1126/sciadv.adw8408. Epub 2025 Jul 25. Sci Adv. 2025. PMID: 40712031 Free PMC article.
-
Catalyst-Controlled Divergent Synthesis of Bicyclo[2.1.1]hexanes and Cyclobutenes: The Unique Effect of Au(I).JACS Au. 2025 May 22;5(6):2738-2748. doi: 10.1021/jacsau.5c00341. eCollection 2025 Jun 23. JACS Au. 2025. PMID: 40575301 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

