Differential gene expression in decidualized human endometrial stromal cells induced by different stimuli
- PMID: 38565619
- PMCID: PMC10987566
- DOI: 10.1038/s41598-024-58065-z
Differential gene expression in decidualized human endometrial stromal cells induced by different stimuli
Abstract
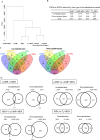
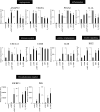
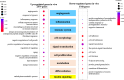
Decidualization can be induced by culturing human endometrial stromal cells (ESCs) with several decidualization stimuli, such as cAMP, medroxyprogesterone acetate (MPA) or Estradiol (E2). However, it has been unclear how decidualized cells induced by different stimuli are different. We compared transcriptomes and cellular functions of decidualized ESCs induced by different stimuli (MPA, E2 + MPA, cAMP, and cAMP + MPA). We also investigated which decidualization stimulus induces a closer in vivo decidualization. Differentially expressed genes (DEGs) and altered cellular functions by each decidualization stimuli were identified by RNA-sequence and gene-ontology analysis. DEGs was about two times higher for stimuli that use cAMP (cAMP and cAMP + MPA) than for stimuli that did not use cAMP (MPA and E2 + MPA). cAMP-using stimuli altered the cellular functions including angiogenesis, inflammation, immune system, and embryo implantation whereas MPA-using stimuli (MPA, E2 + MPA, and cAMP + MPA) altered the cellular functions associated with insulin signaling. A public single-cell RNA-sequence data of the human endometrium was utilized to analyze in vivo decidualization. The altered cellular functions by in vivo decidualization were close to those observed by cAMP + MPA-induced decidualization. In conclusion, decidualized cells induced by different stimuli have different transcriptome and cellular functions. cAMP + MPA may induce a decidualization most closely to in vivo decidualization.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Enhanced ZBTB16 Levels by Progestin-Only Contraceptives Induces Decidualization and Inflammation.Int J Mol Sci. 2023 Jun 23;24(13):10532. doi: 10.3390/ijms241310532. Int J Mol Sci. 2023. PMID: 37445713 Free PMC article.
-
cAMP regulates the progesterone receptor gene expression through the protein kinase A pathway during decidualization in human immortalized endometrial stromal cells.Steroids. 2024 Mar;203:109363. doi: 10.1016/j.steroids.2024.109363. Epub 2024 Jan 4. Steroids. 2024. PMID: 38182066
-
Genome-wide DNA methylation analysis revealed stable DNA methylation status during decidualization in human endometrial stromal cells.BMC Genomics. 2019 Apr 29;20(1):324. doi: 10.1186/s12864-019-5695-0. BMC Genomics. 2019. PMID: 31035926 Free PMC article.
-
Effect of cyclic AMP and estrogen/progesterone on the transcription of DNA methyltransferases during the decidualization of human endometrial stromal cells.Mol Hum Reprod. 2013 May;19(5):302-12. doi: 10.1093/molehr/gas062. Epub 2012 Dec 10. Mol Hum Reprod. 2013. PMID: 23233487
-
Danazol regulates the functions of normal human endometrial stromal cell subpopulations by modifying endometrial cytokine networks.Int J Mol Med. 2009 Mar;23(3):421-8. doi: 10.3892/ijmm_00000147. Int J Mol Med. 2009. PMID: 19212662
Cited by
-
Targeting Cellular Senescence to Enhance Human Endometrial Stromal Cell Decidualization and Inhibit Their Migration.Biomolecules. 2025 Jun 16;15(6):873. doi: 10.3390/biom15060873. Biomolecules. 2025. PMID: 40563513 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

